Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration
- PMID: 20144785
- PMCID: PMC2846417
- DOI: 10.1016/j.stem.2009.12.015
Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration
Abstract
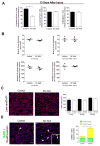
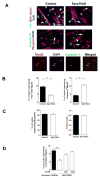
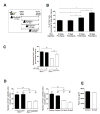
Satellite cells are skeletal muscle stem cells capable of self-renewal and differentiation after transplantation, but whether they contribute to endogenous muscle fiber repair has been unclear. The transcription factor Pax7 marks satellite cells and is critical for establishing the adult satellite cell pool. By using a lineage tracing approach, we show that after injury, quiescent adult Pax7(+) cells enter the cell cycle; a subpopulation returns to quiescence to replenish the satellite cell compartment, while others contribute to muscle fiber formation. We demonstrate that Sprouty1 (Spry1), a receptor tyrosine kinase signaling inhibitor, is expressed in quiescent Pax7(+) satellite cells in uninjured muscle, downregulated in proliferating myogenic cells after injury, and reinduced as Pax7(+) cells re-enter quiescence. We show that Spry1 is required for the return to quiescence and homeostasis of the satellite cell pool during repair. Our results therefore define a role for Spry1 in adult muscle stem cell biology and tissue repair.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
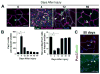
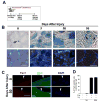
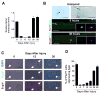
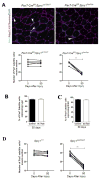
Similar articles
-
Muscle stem cells and reversible quiescence: the role of sprouty.Cell Cycle. 2010 Jul 1;9(13):2575-80. doi: 10.4161/cc.9.13.12149. Cell Cycle. 2010. PMID: 20581433 Review.
-
The aged niche disrupts muscle stem cell quiescence.Nature. 2012 Oct 18;490(7420):355-60. doi: 10.1038/nature11438. Epub 2012 Sep 26. Nature. 2012. PMID: 23023126 Free PMC article.
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
-
CCAAT/enhancer binding protein β is required for satellite cell self-renewal.Skelet Muscle. 2016 Dec 7;6(1):40. doi: 10.1186/s13395-016-0112-8. Skelet Muscle. 2016. PMID: 27923399 Free PMC article.
-
Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements.Nature. 2009 Jul 30;460(7255):627-31. doi: 10.1038/nature08209. Nature. 2009. PMID: 19554048 Free PMC article.
Cited by
-
MSTN Regulatory Network in Mongolian Horse Muscle Satellite Cells Revealed with miRNA Interference Technologies.Genes (Basel). 2022 Oct 11;13(10):1836. doi: 10.3390/genes13101836. Genes (Basel). 2022. PMID: 36292721 Free PMC article.
-
Effects of Hypoxia on Proliferation and Differentiation in Belgian Blue and Hanwoo Muscle Satellite Cells for the Development of Cultured Meat.Biomolecules. 2022 Jun 16;12(6):838. doi: 10.3390/biom12060838. Biomolecules. 2022. PMID: 35740963 Free PMC article.
-
Induction of muscle stem cell quiescence by the secreted niche factor Oncostatin M.Nat Commun. 2018 Apr 18;9(1):1531. doi: 10.1038/s41467-018-03876-8. Nat Commun. 2018. PMID: 29670077 Free PMC article.
-
Divergent Roles of Inflammation in Skeletal Muscle Recovery From Injury.Front Physiol. 2020 Feb 13;11:87. doi: 10.3389/fphys.2020.00087. eCollection 2020. Front Physiol. 2020. PMID: 32116792 Free PMC article. Review.
-
A novel cell-cycle-indicator, mVenus-p27K-, identifies quiescent cells and visualizes G0-G1 transition.Sci Rep. 2014 Feb 6;4:4012. doi: 10.1038/srep04012. Sci Rep. 2014. PMID: 24500246 Free PMC article.
References
-
- Abou-Khalil R, Le GF, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. - PMC - PubMed
-
- Allen RE, Dodson MV, Luiten LS. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp Cell Res. 1984;152:154–160. - PubMed
-
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. - PubMed
-
- Bockhold KJ, Rosenblatt JD, Partridge TA. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve. 1998;21:173–183. - PubMed
-
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

