Rational design of dualsteric GPCR ligands: quests and promise
- PMID: 20136835
- PMCID: PMC2839259
- DOI: 10.1111/j.1476-5381.2009.00601.x
Rational design of dualsteric GPCR ligands: quests and promise
Abstract
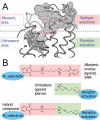
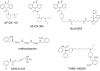
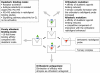
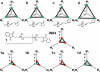
Dualsteric ligands represent a novel mode of targeting G protein-coupled receptors (GPCRs). These compounds attach simultaneously to both, the orthosteric transmitter binding site and an additional allosteric binding area of a receptor protein. This approach allows the exploitation of favourable characteristics of the orthosteric and the allosteric site by a single ligand molecule. The orthosteric interaction provides high affinity binding and activation of receptors. The allosteric interaction yields receptor subtype-selectivity and, in addition, may modulate both, efficacy and intracellular signalling pathway activation. Insight into the spatial arrangement of the orthosteric and the allosteric site is far advanced in the muscarinic acetylcholine receptor, and the design of dualsteric muscarinic agonists has now been accomplished. Using the muscarinic receptor as a paradigm, this review summarizes the way from suggestive evidence for an orthosteric/allosteric overlap binding to the rational design and experimental validation of dualsteric ligands. As allosteric interactions are increasingly described for GPCRs and as insight into the spatial geometry of ligand/GPCR-complexes is growing impressively, the rational design of dualsteric drugs is a promising new approach to achieve fine-tuned GPCR-modulation.
Figures

Similar articles
-
Molecular alliance-from orthosteric and allosteric ligands to dualsteric/bitopic agonists at G protein coupled receptors.Angew Chem Int Ed Engl. 2013 Jan 7;52(2):508-16. doi: 10.1002/anie.201205315. Epub 2012 Dec 6. Angew Chem Int Ed Engl. 2013. PMID: 23225228 Review.
-
Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity.FASEB J. 2009 Feb;23(2):442-50. doi: 10.1096/fj.08-114751. Epub 2008 Oct 8. FASEB J. 2009. PMID: 18842964
-
Recent Advances in the Drug Discovery and Development of Dualsteric/ Bitopic Activators of G Protein-Coupled Receptors.Curr Top Med Chem. 2019;19(26):2378-2392. doi: 10.2174/1568026619666191009164609. Curr Top Med Chem. 2019. PMID: 31833462 Review.
-
Allosteric ligands for G protein-coupled receptors: a novel strategy with attractive therapeutic opportunities.Med Res Rev. 2010 May;30(3):463-549. doi: 10.1002/med.20166. Med Res Rev. 2010. PMID: 19557759 Review.
-
Dualsteric GPCR targeting and functional selectivity: the paradigmatic M(2) muscarinic acetylcholine receptor.Drug Discov Today Technol. 2013 Summer;10(2):e245-52. doi: 10.1016/j.ddtec.2012.12.003. Drug Discov Today Technol. 2013. PMID: 24050275 Review.
Cited by
-
Novel Xanomeline-Containing Bitopic Ligands of Muscarinic Acetylcholine Receptors: Design, Synthesis and FRET Investigation.Molecules. 2023 Mar 6;28(5):2407. doi: 10.3390/molecules28052407. Molecules. 2023. PMID: 36903650 Free PMC article.
-
Drugs for allosteric sites on receptors.Annu Rev Pharmacol Toxicol. 2014;54:165-84. doi: 10.1146/annurev-pharmtox-010611-134525. Epub 2013 Oct 2. Annu Rev Pharmacol Toxicol. 2014. PMID: 24111540 Free PMC article. Review.
-
Selective inhibitors of mTORC1 activate 4EBP1 and suppress tumor growth.Nat Chem Biol. 2021 Oct;17(10):1065-1074. doi: 10.1038/s41589-021-00813-7. Epub 2021 Jun 24. Nat Chem Biol. 2021. PMID: 34168367 Free PMC article.
-
Novel Allosteric Modulators of G Protein-coupled Receptors.J Biol Chem. 2015 Aug 7;290(32):19478-88. doi: 10.1074/jbc.R115.662759. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100627 Free PMC article. Review.
-
A structural chemogenomics analysis of aminergic GPCRs: lessons for histamine receptor ligand design.Br J Pharmacol. 2013 Sep;170(1):101-26. doi: 10.1111/bph.12248. Br J Pharmacol. 2013. PMID: 23713847 Free PMC article.
References
-
- Antony J, Kellershohn K, Mohr-Andrä M, Kebig A, Prilla S, Muth M, et al. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 2009;23:442–450. - PubMed
-
- Avlani VA, Gregory KJ, Morton CJ, Parker MW, Sexton PM, Christopoulos A. Critical role for the second extracellular loop in the binding of both orthosteric and allosteric G protein-coupled receptor ligands. J Biol Chem. 2007;282:25677–25686. - PubMed
-
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428.
-
- Birdsall NJ, Lazareno S. Allosterism at muscarinic receptors: ligands and mechanisms. Mini Rev Med Chem. 2005;5:523–543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

