Dietary zinc and prostate cancer in the TRAMP mouse model
- PMID: 20136438
- PMCID: PMC2991100
- DOI: 10.1089/jmf.2009.0042
Dietary zinc and prostate cancer in the TRAMP mouse model
Abstract
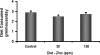
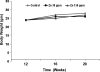
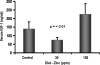
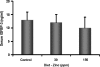
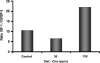
Circumstantial evidence indicates that zinc may have an important role in the prostate. Total zinc levels in the prostate are 10 times higher than in other soft tissues. Zinc concentrations in prostate epithethial cancer cells are decreased significantly. Zinc supplementation for prevention and treatment of prostate cancer in humans has yielded controversial results. No studies have been reported in animal models to show the effect of zinc supplementation on prevention of prostate cancer, thus far. In this study, we have examined the effect of zinc supplementation on development of prostate cancer in a TRAMP mouse model. Results from our study indicate that dietary zinc plays an important role in prostate carcinogenesis. Tumor weights were significantly higher when the dietary zinc intake was either deficient or high in comparison to normal zinc intake level, suggesting that an optimal dietary zinc intake may play a protective role against prostate cancer. Further, our studies also showed decreased insulin-like growth factor (IGF)-1 and IGF-1/IGF binding protein-3 ratio in normal zinc-supplemented animals, suggesting that zinc may modulate IGF-1 metabolism in relation to carcinogenesis. We conclude that optimal prostate zinc concentration has a protective role against cancer.
Figures
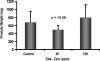
Similar articles
-
Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression.Cancer Res. 2008 May 1;68(9):3342-9. doi: 10.1158/0008-5472.CAN-07-3165. Cancer Res. 2008. PMID: 18451161 Free PMC article.
-
The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model.Cancer Res. 1999 May 1;59(9):2203-9. Cancer Res. 1999. PMID: 10232609
-
Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice.Prostate. 2014 Dec;74(16):1663-73. doi: 10.1002/pros.22884. Epub 2014 Sep 22. Prostate. 2014. PMID: 25250521
-
Insulin-like growth factors and prostate cancer.Cancer Metastasis Rev. 1998-1999;17(4):383-90. doi: 10.1023/a:1006154108619. Cancer Metastasis Rev. 1998. PMID: 10453282 Review.
-
Prostate cancer prevention by nutritional means to alleviate metabolic syndrome.Am J Clin Nutr. 2007 Sep;86(3):s889-93. doi: 10.1093/ajcn/86.3.889S. Am J Clin Nutr. 2007. PMID: 18265484 Review.
Cited by
-
The association between zinc and prostate cancer development: A systematic review and meta-analysis.PLoS One. 2024 Mar 20;19(3):e0299398. doi: 10.1371/journal.pone.0299398. eCollection 2024. PLoS One. 2024. PMID: 38507438 Free PMC article.
-
Impact of dietary zinc on stimulated zinc secretion MRI in the healthy and malignant mouse prostate.Npj Imaging. 2024;2(1):47. doi: 10.1038/s44303-024-00051-1. Epub 2024 Nov 6. Npj Imaging. 2024. PMID: 39525279 Free PMC article.
-
Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review.Nutrients. 2024 Jun 26;16(13):2026. doi: 10.3390/nu16132026. Nutrients. 2024. PMID: 38999774 Free PMC article. Review.
-
Zinc and zinc transporters in prostate carcinogenesis.Nat Rev Urol. 2013 Apr;10(4):219-26. doi: 10.1038/nrurol.2013.43. Epub 2013 Mar 12. Nat Rev Urol. 2013. PMID: 23478540 Free PMC article. Review.
-
Zinc dampens antitumor immunity by promoting Foxp3+ regulatory T cells.Front Immunol. 2024 Aug 23;15:1389387. doi: 10.3389/fimmu.2024.1389387. eCollection 2024. Front Immunol. 2024. PMID: 39247196 Free PMC article.
References
-
- Prasad AS. Plenum Press; New York: 1993. Biochemistry of Zinc; pp. 219–258.
-
- Prasad AS. Zinc: mechanism of host defense. J Nutr. 2007;137:1345–1349. - PubMed
-
- Prasad AS. Miale A., Jr Farid Z. Sandstead HH. Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J Lab Clin Med. 1963;61:537–549. - PubMed
-
- Sandstead HH. Prasad AS. Schulert AR. Farid Z. Miale A. Bassily S. Darby WJ. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–442. - PubMed
-
- O'Dell BL. Burpo CE. Savage JE. Evaluation of zinc availability in foodstuffs of plant and animal origin. J Nutr. 1972;102:653–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

