Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation
- PMID: 20133698
- PMCID: PMC2840351
- DOI: 10.1073/pnas.0913035107
Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation
Abstract
The productivity of higher plants as a major source of food and energy is linked to their ability to buffer changes in the concentrations of essential and toxic ions. Transport across the tonoplast is energized by two proton pumps, the vacuolar H(+)-ATPase (V-ATPase) and the vacuolar H(+)-pyrophosphatase (V-PPase); however, their functional relation and relative contributions to ion storage and detoxification are unclear. We have identified an Arabidopsis mutant in which energization of vacuolar transport solely relies on the activity of the V-PPase. The vha-a2 vha-a3 double mutant, which lacks the two tonoplast-localized isoforms of the membrane-integral V-ATPase subunit VHA-a, is viable but shows day-length-dependent growth retardation. Nitrate content is reduced whereas nitrate assimilation is increased in the vha-a2 vha-a3 mutant, indicating that vacuolar nitrate storage represents a major growth-limiting factor. Zinc is an essential micronutrient that is toxic at excess concentrations and is detoxified via a vacuolar Zn(2+)/H(+)-antiport system. Accordingly, the double mutant shows reduced zinc tolerance. In the same way the vacuolar Na(+)/H(+)-antiport system is assumed to be an important component of the system that removes sodium from the cytosol. Unexpectedly, salt tolerance and accumulation are not affected in the vha-a2 vha-a3 double mutant. In contrast, reduction of V-ATPase activity in the trans-Golgi network/early endosome (TGN/EE) leads to increased salt sensitivity. Taken together, our results show that during gametophyte and embryo development V-PPase activity at the tonoplast is sufficient whereas tonoplast V-ATPase activity is limiting for nutrient storage but not for sodium tolerance during vegetative and reproductive growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
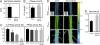
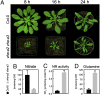

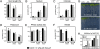
Similar articles
-
Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana.Int J Mol Sci. 2020 Dec 22;22(1):2. doi: 10.3390/ijms22010002. Int J Mol Sci. 2020. PMID: 33374906 Free PMC article.
-
Job Sharing in the Endomembrane System: Vacuolar Acidification Requires the Combined Activity of V-ATPase and V-PPase.Plant Cell. 2015 Dec;27(12):3383-96. doi: 10.1105/tpc.15.00733. Epub 2015 Nov 20. Plant Cell. 2015. PMID: 26589552 Free PMC article.
-
A Defective Vacuolar Proton Pump Enhances Aluminum Tolerance by Reducing Vacuole Sequestration of Organic Acids.Plant Physiol. 2019 Oct;181(2):743-761. doi: 10.1104/pp.19.00626. Epub 2019 Jul 26. Plant Physiol. 2019. PMID: 31350362 Free PMC article.
-
Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange.Plant Signal Behav. 2009 Aug;4(8):718-26. doi: 10.4161/psb.4.8.9236. Epub 2009 Aug 9. Plant Signal Behav. 2009. PMID: 19820346 Free PMC article. Review.
-
Biochemical, Structural and Physiological Characteristics of Vacuolar H+-Pyrophosphatase.Plant Cell Physiol. 2018 Jul 1;59(7):1300-1308. doi: 10.1093/pcp/pcy054. Plant Cell Physiol. 2018. PMID: 29534212 Review.
Cited by
-
Arabidopsis COP1 guides stomatal response in guard cells through pH regulation.Commun Biol. 2024 Feb 5;7(1):150. doi: 10.1038/s42003-024-05847-w. Commun Biol. 2024. PMID: 38316905 Free PMC article.
-
Subcellular Localization and Vesicular Structures of Anthocyanin Pigmentation by Fluorescence Imaging of Black Rice (Oryza sativa L.) Stigma Protoplast.Plants (Basel). 2021 Apr 2;10(4):685. doi: 10.3390/plants10040685. Plants (Basel). 2021. PMID: 33918111 Free PMC article.
-
Enhanced expression of vacuolar H+-ATPase subunit E in the roots is associated with the adaptation of Broussonetia papyrifera to salt stress.PLoS One. 2012;7(10):e48183. doi: 10.1371/journal.pone.0048183. Epub 2012 Oct 25. PLoS One. 2012. PMID: 23133565 Free PMC article.
-
Regulation of vacuolar H+-ATPase activity by the Cdc42 effector Ste20 in Saccharomyces cerevisiae.Eukaryot Cell. 2012 Apr;11(4):442-51. doi: 10.1128/EC.05286-11. Epub 2012 Feb 10. Eukaryot Cell. 2012. PMID: 22327006 Free PMC article.
-
Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in Citrus leaves.BMC Plant Biol. 2018 Sep 12;18(1):188. doi: 10.1186/s12870-018-1413-3. BMC Plant Biol. 2018. PMID: 30208853 Free PMC article.
References
-
- Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. J Exp Bot. 2007;58:83–102. - PubMed
-
- Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–2306. - PubMed
-
- De Angeli A, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

