Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years
- PMID: 20126454
- PMCID: PMC2813284
- DOI: 10.1371/journal.pone.0008809
Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years
Abstract
Background: Essentially all knowledge about adult hippocampal neurogenesis in humans still comes from one seminal study by Eriksson et al. in 1998, although several others have provided suggestive findings. But only little information has been available in how far the situation in animal models would reflect the conditions in the adult and aging human brain. We therefore here mapped numerous features associated with adult neurogenesis in rodents in samples from human hippocampus across the entire lifespan. Such data would not offer proof of adult neurogenesis in humans, because it is based on the assumption that humans and rodents share marker expression patterns in adult neurogenesis. Nevertheless, together the data provide valuable information at least about the presence of markers, for which a link to adult neurogenesis might more reasonably be assumed than for others, in the adult human brain and their change with increasing age.
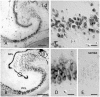
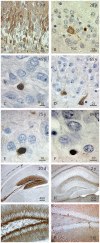
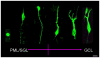
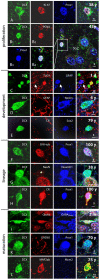
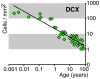
Methods and findings: In rodents, doublecortin (DCX) is transiently expressed during adult neurogenesis and within the neurogenic niche of the dentate gyrus can serve as a valuable marker. We validated DCX as marker of granule cell development in fetal human tissue and used DCX expression as seed to examine the dentate gyrus for additional neurogenesis-associated features across the lifespan. We studied 54 individuals and detected DCX expression between birth and 100 years of age. Caveats for post-mortem analyses of human tissues apply but all samples were free of signs of ischemia and activated caspase-3. Fourteen markers related to adult hippocampal neurogenesis in rodents were assessed in DCX-positive cells. Total numbers of DCX expressing cells declined exponentially with increasing age, and co-expression of DCX with the other markers decreased. This argued against a non-specific re-appearance of immature markers in specimen from old brains. Early postnatally all 14 markers were co-expressed in DCX-positive cells. Until 30 to 40 years of age, for example, an overlap of DCX with Ki67, Mcm2, Sox2, Nestin, Prox1, PSA-NCAM, Calretinin, NeuN, and others was detected, and some key markers (Nestin, Sox2, Prox1) remained co-expressed into oldest age.
Conclusions: Our data suggest that in the adult human hippocampus neurogenesis-associated features that have been identified in rodents show patterns, as well as qualitative and quantitative age-related changes, that are similar to the course of adult hippocampal neurogenesis in rodents. Consequently, although further validation as well as the application of independent methodology (e.g. electron microscopy and cell culture work) is desirable, our data will help to devise the framework for specific research on cellular plasticity in the aging human hippocampus.
Conflict of interest statement
Figures




Similar articles
-
Characterization of dsRed2-positive cells in the doublecortin-dsRed2 transgenic adult rat retina.Histochem Cell Biol. 2014 Dec;142(6):601-17. doi: 10.1007/s00418-014-1259-1. Epub 2014 Aug 20. Histochem Cell Biol. 2014. PMID: 25138677
-
Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice.PLoS One. 2011;6(10):e25760. doi: 10.1371/journal.pone.0025760. Epub 2011 Oct 13. PLoS One. 2011. PMID: 22022443 Free PMC article.
-
Doublecortin-expressing cell types in temporal lobe epilepsy.Acta Neuropathol Commun. 2018 Jul 13;6(1):60. doi: 10.1186/s40478-018-0566-5. Acta Neuropathol Commun. 2018. PMID: 30005693 Free PMC article.
-
Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus.J Neurosci. 2021 Mar 24;41(12):2554-2565. doi: 10.1523/JNEUROSCI.0676-20.2020. J Neurosci. 2021. PMID: 33762407 Free PMC article. Review.
-
Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus.Cell Tissue Res. 2011 Jul;345(1):1-19. doi: 10.1007/s00441-011-1196-4. Epub 2011 Jun 7. Cell Tissue Res. 2011. PMID: 21647561 Review.
Cited by
-
The CRISP theory of hippocampal function in episodic memory.Front Neural Circuits. 2013 May 6;7:88. doi: 10.3389/fncir.2013.00088. eCollection 2013. Front Neural Circuits. 2013. PMID: 23653597 Free PMC article. Review.
-
Adult Neurogenesis and Gliogenesis: Possible Mechanisms for Neurorestoration.Exp Neurobiol. 2016 Jun;25(3):103-12. doi: 10.5607/en.2016.25.3.103. Epub 2016 Jun 16. Exp Neurobiol. 2016. PMID: 27358578 Free PMC article. Review.
-
Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory.Brain Res. 2016 Aug 1;1644:127-40. doi: 10.1016/j.brainres.2016.05.015. Epub 2016 May 10. Brain Res. 2016. PMID: 27174001 Free PMC article. Review.
-
Is Adult Hippocampal Neurogenesis Really Relevant for the Treatment of Psychiatric Disorders?Curr Neuropharmacol. 2021;19(10):1640-1660. doi: 10.2174/1570159X18666200818194948. Curr Neuropharmacol. 2021. PMID: 32811415 Free PMC article. Review.
-
Wnt signaling in the regulation of adult hippocampal neurogenesis.Front Cell Neurosci. 2013 Jun 26;7:100. doi: 10.3389/fncel.2013.00100. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23805076 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

