Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation
- PMID: 20118982
- PMCID: PMC3287039
- DOI: 10.1038/onc.2009.521
Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation
Erratum in
- Oncogene. 2011 Apr 14;30(15):1850
Abstract
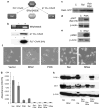
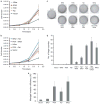
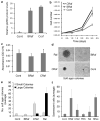
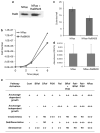
Cutaneous malignant melanoma is considered one of the most deadly human cancers, based on both its penchant for metastatic spread and its typical resistance to currently available therapy. Long known to harbor oncogenic NRAS mutations, melanomas were more recently reported to be frequent bearers of activating mutations in BRAF, one of the effectors situated downstream of wild-type NRAS. NRAS and BRAF mutations are rarely found in the same melanoma, suggesting that they may possess important overlapping oncogenic activities. Here, we compare and contrast the oncogenic roles of the three major NRas downstream effectors, Raf, phosphatidylinositol 3-kinase (PI3K) and Ral guanine exchange factor (RalGEF), using genetically engineered Arf-deficient immortalized mouse melanocytes as a model system. Although no single downstream pathway could recapitulate all of the consequences of oncogenic NRas expression, our data indicate a prominent role for BRaf and PI3K in melanocyte senescence and invasiveness, respectively. More surprisingly, we discovered that constitutive RalGEF activation had a major impact on several malignant phenotypes, particularly anchorage-independent growth, indicating that this often overlooked pathway should be more carefully evaluated as a possible therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ral activation promotes melanomagenesis.Oncogene. 2010 Aug 26;29(34):4859-64. doi: 10.1038/onc.2010.224. Epub 2010 Jun 21. Oncogene. 2010. PMID: 20562921 Free PMC article.
-
Modulation of phospholipase D by Ras proteins mediated by its effectors Ral-GDS, PI3K and Raf-1.Int J Oncol. 2002 Sep;21(3):477-85. Int J Oncol. 2002. PMID: 12168089
-
C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells.Oncogene. 2008 Nov 6;27(52):6623-34. doi: 10.1038/onc.2008.258. Epub 2008 Aug 4. Oncogene. 2008. PMID: 18679422 Free PMC article.
-
Driver mutations in melanoma: lessons learned from bench-to-bedside studies.Curr Oncol Rep. 2012 Oct;14(5):449-57. doi: 10.1007/s11912-012-0249-5. Curr Oncol Rep. 2012. PMID: 22723080 Free PMC article. Review.
-
Regulation of Ras signaling by the cell cycle.Curr Opin Genet Dev. 2002 Feb;12(1):44-6. doi: 10.1016/s0959-437x(01)00262-3. Curr Opin Genet Dev. 2002. PMID: 11790553 Review.
Cited by
-
Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail.Biochem Pharmacol. 2010 Sep 1;80(5):624-37. doi: 10.1016/j.bcp.2010.04.029. Epub 2010 May 9. Biochem Pharmacol. 2010. PMID: 20450891 Free PMC article. Review.
-
Beyond BRAF: where next for melanoma therapy?Br J Cancer. 2015 Jan 20;112(2):217-26. doi: 10.1038/bjc.2014.476. Epub 2014 Sep 2. Br J Cancer. 2015. PMID: 25180764 Free PMC article. Review.
-
The RalGEF-Ral Effector Signaling Network: The Road Less Traveled for Anti-Ras Drug Discovery.Genes Cancer. 2011 Mar;2(3):275-87. doi: 10.1177/1947601911407329. Genes Cancer. 2011. PMID: 21779498 Free PMC article.
-
Molecular pathways: targeting NRAS in melanoma and acute myelogenous leukemia.Clin Cancer Res. 2014 Aug 15;20(16):4186-92. doi: 10.1158/1078-0432.CCR-13-3270. Epub 2014 Jun 3. Clin Cancer Res. 2014. PMID: 24895460 Free PMC article. Review.
-
Ral small GTPase signaling and oncogenesis: More than just 15minutes of fame.Biochim Biophys Acta. 2014 Dec;1843(12):2976-2988. doi: 10.1016/j.bbamcr.2014.09.004. Epub 2014 Sep 16. Biochim Biophys Acta. 2014. PMID: 25219551 Free PMC article. Review.
References
-
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. - PubMed
-
- Bennett DC. How to make a melanoma: what do we know of the primary clonal events? Pig Cell Melan Res. 2008;21:27–38. - PubMed
-
- Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev. 2008;8:133–140. - PubMed
-
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. - PubMed
-
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

