Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study
- PMID: 20112381
- PMCID: PMC3057537
- DOI: 10.1002/art.27221
Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study
Abstract
Objective: To assess the safety of interleukin-6 receptor inhibition and to collect preliminary data on the clinical and immunologic efficacy of tocilizumab in patients with systemic lupus erythematosus (SLE).
Methods: In an open-label phase I dosage-escalation study, 16 patients with mild-to-moderate disease activity were assigned to receive 1 of 3 doses of tocilizumab given intravenously every other week for 12 weeks (total of 7 infusions): 2 mg/kg in 4 patients, 4 mg/kg in 6 patients, or 8 mg/kg in 6 patients. Patients were then monitored for an additional 8 weeks.
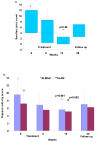
Results: The infusions were well tolerated. Tocilizumab treatment led to dosage-related decreases in the absolute neutrophil count, with a median decrease of 38% in the 4 mg/kg dosage group and 56% in the 8 mg/kg dosage group. Neutrophil counts returned to normal after cessation of treatment. One patient was withdrawn from the study because of neutropenia. Infections occurred in 11 patients; none was associated with neutropenia. Disease activity showed significant improvement, with a decrease of > or =4 points in the modified Safety of Estrogens in Lupus Erythematosus National Assessment version of the Systemic Lupus Erythematosus Disease Activity Index score in 8 of the 15 evaluable patients. Arthritis improved in all 7 patients who had arthritis at baseline and resolved in 4 of them. Levels of anti-double-stranded DNA antibodies decreased by a median of 47% in patients in the 4 mg/kg and 8 mg/kg dosage groups, with a 7.8% decrease in their IgG levels. These changes, together with a significant decrease in the frequency of circulating plasma cells, suggest a specific effect of tocilizumab on autoantibody-producing cells.
Conclusion: Although neutropenia may limit the maximum dosage of tocilizumab in patients with SLE, the observed clinical and serologic responses are promising and warrant further studies to establish the optimal dosing regimen and efficacy.
Trial registration: ClinicalTrials.gov NCT00046774.
Figures



Similar articles
-
Phase I, randomized, double-blind, placebo-controlled, multiple intravenous, dose-ascending study of sirukumab in cutaneous or systemic lupus erythematosus.Arthritis Rheum. 2013 Oct;65(10):2661-71. doi: 10.1002/art.38091. Arthritis Rheum. 2013. PMID: 23896980 Clinical Trial.
-
Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial.Ann Rheum Dis. 2018 Jun;77(6):883-889. doi: 10.1136/annrheumdis-2018-213032. Epub 2018 Mar 21. Ann Rheum Dis. 2018. PMID: 29563108 Clinical Trial.
-
A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus.Arthritis Rheum. 2009 Sep 15;61(9):1168-78. doi: 10.1002/art.24699. Arthritis Rheum. 2009. PMID: 19714604 Free PMC article. Clinical Trial.
-
Tocilizumab: a review of its use in the management of rheumatoid arthritis.Drugs. 2009;69(5):609-32. doi: 10.2165/00003495-200969050-00007. Drugs. 2009. PMID: 19368420 Review.
-
Belimumab: review of use in systemic lupus erythematosus.Clin Ther. 2012 May;34(5):1006-22. doi: 10.1016/j.clinthera.2012.02.028. Epub 2012 Mar 30. Clin Ther. 2012. PMID: 22464040 Review.
Cited by
-
Analysis of gene expression from systemic lupus erythematosus synovium reveals myeloid cell-driven pathogenesis of lupus arthritis.Sci Rep. 2020 Oct 15;10(1):17361. doi: 10.1038/s41598-020-74391-4. Sci Rep. 2020. PMID: 33060686 Free PMC article.
-
Ten developments in the use of biologicals for systemic lupus erythematosus.Curr Rheumatol Rep. 2013 Jul;15(7):337. doi: 10.1007/s11926-013-0337-z. Curr Rheumatol Rep. 2013. PMID: 23666467 Review.
-
Successful treatment of recurrent pleural and pericardial effusions with tocilizumab in a patient with systemic lupus erythematous.BMJ Case Rep. 2016 Aug 8;2016:bcr2016215423. doi: 10.1136/bcr-2016-215423. BMJ Case Rep. 2016. PMID: 27503940 Free PMC article.
-
IVIg attenuates TLR-9 activation in B cells from SLE patients.J Clin Immunol. 2011 Feb;31(1):30-8. doi: 10.1007/s10875-010-9469-3. Epub 2010 Oct 5. J Clin Immunol. 2011. PMID: 20922561
-
The role of cytokine in the lupus nephritis.J Biomed Biotechnol. 2011;2011:594809. doi: 10.1155/2011/594809. Epub 2011 Oct 18. J Biomed Biotechnol. 2011. PMID: 22028590 Free PMC article. Review.
References
-
- Kishimoto T. IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. 2005;28(3):177–86. - PubMed
-
- Kobayashi I, Matsuda T, Saito T, Yasukawa K, Kikutani H, Hirano T, et al. Abnormal distribution of IL-6 receptor in aged MRL/lpr mice: elevated expression on B cells and absence on CD4+ cells. Int Immunol. 1992;4(12):1407–1412. - PubMed
-
- Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, et al. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur J Immunol. 1993;23(5):1078–1082. - PubMed
-
- Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, et al. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3(3):273–278. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

