VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD
- PMID: 20104022
- PMCID: PMC2929010
- DOI: 10.4161/auto.6.2.11014
VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD
Abstract
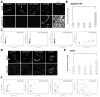
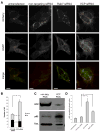
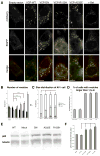
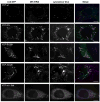
VCP (VCP/p97) is a ubiquitously expressed member of the AAA(+)-ATPase family of chaperone-like proteins that regulates numerous cellular processes including chromatin decondensation, homotypic membrane fusion and ubiquitin-dependent protein degradation by the proteasome. Mutations in VCP cause a multisystem degenerative disease consisting of inclusion body myopathy, Paget disease of bone, and frontotemporal dementia (IBMPFD). Here we show that VCP is essential for autophagosome maturation. We generated cells stably expressing dual-tagged LC3 (mCherry-EGFP-LC3) which permit monitoring of autophagosome maturation. We determined that VCP deficiency by RNAi-mediated knockdown or overexpression of dominant-negative VCP results in significant accumulation of immature autophagic vesicles, some of which are abnormally large, acidified and exhibit cathepsin B activity. Furthermore, expression of disease-associated VCP mutants (R155H and A232E) also causes this autophagy defect. VCP was found to be essential to autophagosome maturation under basal conditions and in cells challenged by proteasome inhibition, but not in cells challenged by starvation, suggesting that VCP might be selectively required for autophagic degradation of ubiquitinated substrates. Indeed, a high percentage of the accumulated autophagic vesicles contain ubiquitin-positive contents, a feature that is not observed in autophagic vesicles that accumulate following starvation or treatment with Bafilomycin A. Finally, we show accumulation of numerous, large LAMP-1 and LAMP-2-positive vacuoles and accumulation of LC3-II in myoblasts derived from patients with IBMPFD. We conclude that VCP is essential for maturation of ubiquitin-containing autophagosomes and that defect in this function may contribute to IBMPFD pathogenesis.
Figures
Similar articles
-
Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease.J Cell Biol. 2009 Dec 14;187(6):875-88. doi: 10.1083/jcb.200908115. J Cell Biol. 2009. PMID: 20008565 Free PMC article.
-
Inclusion body myopathy, Paget's disease of the bone and fronto-temporal dementia: a disorder of autophagy.Hum Mol Genet. 2010 Apr 15;19(R1):R38-45. doi: 10.1093/hmg/ddq157. Epub 2010 Apr 21. Hum Mol Genet. 2010. PMID: 20410287 Free PMC article. Review.
-
p97/VCP at the intersection of the autophagy and the ubiquitin proteasome system.Autophagy. 2010 Feb;6(2):283-5. doi: 10.4161/auto.6.2.11063. Epub 2010 Mar 1. Autophagy. 2010. PMID: 20083896 Free PMC article. No abstract available.
-
Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation.Hum Mol Genet. 2006 Jan 15;15(2):189-99. doi: 10.1093/hmg/ddi426. Epub 2005 Dec 1. Hum Mol Genet. 2006. PMID: 16321991
-
Valosin-Containing Protein (VCP)/p97 Oligomerization.Subcell Biochem. 2024;104:485-501. doi: 10.1007/978-3-031-58843-3_18. Subcell Biochem. 2024. PMID: 38963497 Review.
Cited by
-
Structure-activity relationship study reveals ML240 and ML241 as potent and selective inhibitors of p97 ATPase.ChemMedChem. 2013 Feb;8(2):297-312. doi: 10.1002/cmdc.201200520. Epub 2013 Jan 11. ChemMedChem. 2013. PMID: 23316025 Free PMC article.
-
Ubiquitin-specific protease 25 functions in Endoplasmic Reticulum-associated degradation.PLoS One. 2012;7(5):e36542. doi: 10.1371/journal.pone.0036542. Epub 2012 May 9. PLoS One. 2012. PMID: 22590560 Free PMC article.
-
Autophagy and neuronal cell death in neurological disorders.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a008839. doi: 10.1101/cshperspect.a008839. Cold Spring Harb Perspect Biol. 2012. PMID: 22983160 Free PMC article. Review.
-
The p97/VCP ATPase is critical in muscle atrophy and the accelerated degradation of muscle proteins.EMBO J. 2012 Aug 1;31(15):3334-50. doi: 10.1038/emboj.2012.178. Epub 2012 Jul 6. EMBO J. 2012. PMID: 22773186 Free PMC article.
-
Proliferating cell nuclear antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis.J Biol Chem. 2012 Oct 5;287(41):34225-33. doi: 10.1074/jbc.M112.400135. Epub 2012 Aug 17. J Biol Chem. 2012. PMID: 22902628 Free PMC article.
References
-
- De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–92. - PubMed
-
- Tooze SA, Razi M. The essential role of early endosomes in autophagy is revealed by loss of COPI function. Autophagy. 2009;5:874–5. - PubMed
-
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. - PubMed
-
- Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci. 2004;117:2687–97. - PubMed
-
- Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
