Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis
- PMID: 20103601
- PMCID: PMC2820597
- DOI: 10.1158/1535-7163.MCT-09-0461
Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis
Abstract
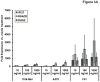
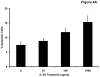
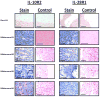
Interleukin-29 (IL-29) is a member of the type III IFN family that has been shown to have antiviral activity and to inhibit cell growth. Melanoma cell lines were tested for expression of the IL-29 receptor (IL-29R) and their response to IL-29. Expression of IL-28R1 and IL-10R2, components of IL-29R, was evaluated using reverse transcription-PCR. A combination of immunoblot analysis and flow cytometry was used to evaluate IL-29-induced signal transduction. U133 Plus 2.0 Arrays and real-time PCR were used to evaluate gene expression. Apoptosis was measured using Annexin V/propridium iodide staining. In situ PCR for IL-29R was done on paraffin-embedded melanoma tumors. Both IL-28R1 and IL-10R2 were expressed on the A375, 1106 MEL, Hs294T, 18105 MEL, MEL 39, SK MEL 5, and F01 cell lines. Incubation of melanoma cell lines with IL-29 (10-1,000 ng/mL) led to phosphorylation of signal transducer and activator of transcription 1 (STAT1) and STAT2. Microarray analysis and quantitative reverse transcription-PCR showed a marked increase in transcripts of IFN-regulated genes after treatment with IL-29. In the F01 cell line, bortezomib-induced and temozolomide-induced apoptosis was synergistically enhanced following the addition of IL-29. In situ PCR revealed that IL-10R2 and IL-28R1 were present in six of eight primary human melanoma tumors but not in benign nevi specimens. In conclusion, IL-29 receptors are expressed on the surface of human melanoma cell lines and patient samples, and treatment of these cell lines with IL-29 leads to signaling via the Jak-STAT pathway, the transcription of a unique set of genes, and apoptosis.
Figures










Similar articles
-
Contribution of the STAT Family of Transcription Factors to the Expression of the Serotonin 2B (HTR2B) Receptor in Human Uveal Melanoma.Int J Mol Sci. 2022 Jan 29;23(3):1564. doi: 10.3390/ijms23031564. Int J Mol Sci. 2022. PMID: 35163491 Free PMC article.
-
Interleukin-24 overcomes temozolomide resistance and enhances cell death by down-regulation of O6-methylguanine-DNA methyltransferase in human melanoma cells.Mol Cancer Ther. 2008 Dec;7(12):3842-51. doi: 10.1158/1535-7163.MCT-08-0516. Epub 2008 Dec 3. Mol Cancer Ther. 2008. PMID: 19056673 Free PMC article.
-
Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells.Clin Cancer Res. 2007 Sep 1;13(17):5010-9. doi: 10.1158/1078-0432.CCR-06-3092. Clin Cancer Res. 2007. PMID: 17785551
-
The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line.Melanoma Res. 2003 Jun;13(3):219-29. doi: 10.1097/00008390-200306000-00001. Melanoma Res. 2003. PMID: 12777975
-
A Comprehensive Network Map of Interleukin-26 Signaling Pathway.J Interferon Cytokine Res. 2024 Sep;44(9):408-413. doi: 10.1089/jir.2024.0026. Epub 2024 Apr 19. J Interferon Cytokine Res. 2024. PMID: 38639111 Review.
Cited by
-
JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens.Front Bioeng Biotechnol. 2023 Feb 23;11:1110765. doi: 10.3389/fbioe.2023.1110765. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36911202 Free PMC article. Review.
-
Identification of a Predominantly Interferon-λ-Induced Transcriptional Profile in Murine Intestinal Epithelial Cells.Front Immunol. 2017 Oct 16;8:1302. doi: 10.3389/fimmu.2017.01302. eCollection 2017. Front Immunol. 2017. PMID: 29085367 Free PMC article.
-
Crystal structure of human interferon-λ1 in complex with its high-affinity receptor interferon-λR1.J Mol Biol. 2010 Dec 10;404(4):650-64. doi: 10.1016/j.jmb.2010.09.068. Epub 2010 Oct 8. J Mol Biol. 2010. PMID: 20934432 Free PMC article.
-
IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-γ production.J Immunol. 2011 Mar 15;186(6):3401-9. doi: 10.4049/jimmunol.1000328. Epub 2011 Feb 14. J Immunol. 2011. PMID: 21321106 Free PMC article.
-
Recombinant Newcastle disease virus expressing human IFN-λ1 (rL-hIFN-λ1) inhibits lung cancer migration through repolarizating macrophage from M2 to M1 phenotype.Transl Cancer Res. 2020 May;9(5):3392-3405. doi: 10.21037/tcr-19-2320. Transl Cancer Res. 2020. PMID: 35117705 Free PMC article.
References
-
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–34. - PubMed
-
- Kim SH, Cohen B, Novick D, Rubinstein M. Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene. 1997;196:279–86. - PubMed
-
- Haque SJ, Williams BR. Signal transduction in the interferon system. Semin Oncol. 1998;25:14–22. - PubMed
-
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA95426/CA/NCI NIH HHS/United States
- K24 CA093670/CA/NCI NIH HHS/United States
- K24 CA93670/CA/NCI NIH HHS/United States
- P01 CA095426-07/CA/NCI NIH HHS/United States
- CA84402/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- T32CA009338/CA/NCI NIH HHS/United States
- P30 CA16058/CA/NCI NIH HHS/United States
- R21 CA084402-02S1/CA/NCI NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- P01 CA095426/CA/NCI NIH HHS/United States
- K22 CA134551/CA/NCI NIH HHS/United States
- K24 CA093670-07/CA/NCI NIH HHS/United States
- T32 CA009338/CA/NCI NIH HHS/United States
- P30 CA016058-29/CA/NCI NIH HHS/United States
- T32 CA009338-28/CA/NCI NIH HHS/United States
- K22 CA134551-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

