The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28
- PMID: 20102549
- PMCID: PMC2974616
- DOI: 10.1111/j.1600-0854.2010.1045.x
The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28
Abstract
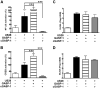
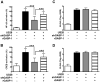
Human cytomegalovirus (HCMV) encodes the seven transmembrane(7TM)/G-protein coupled receptor (GPCR) US28, which signals and endocytoses in a constitutive, ligand-independent manner. Here we show that, following endocytosis, US28 is targeted to the lysosomes for degradation as a consequence of its interaction with the GPCR-associated sorting protein-1 (GASP-1). We find that GASP-1 binds to US28 in vitro and that disruption of the GASP-1/US28 interaction by either (i) overexpression of dominant negative cGASP-1 or by (ii) shRNA knock-down of endogenous GASP-1 is sufficient to inhibit the lysosomal targeting of US28 and slow its post-endocytic degradation. Furthermore, we found that GASP-1 affects US28-mediated signalling. The knock-down of endogenous GASP-1 impairs the US28-mediated Galphaq/PLC/inositol phosphate (IP) accumulation as well as the activation of the transcription factors Nuclear Factor-kappaB (NF-kappaB) and cyclic AMP responsive element binding protein (CREB). Overexpression of GASP-1 enhances both IP accumulation and transcription factor activity. Thus, GASP-1 is an important cellular determinant that not only regulates the post-endocytic trafficking of US28, but also regulates the signalling capacities of US28.
Figures
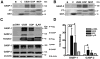




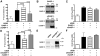
Similar articles
-
The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis.J Biol Chem. 2003 May 23;278(21):19473-82. doi: 10.1074/jbc.M213179200. Epub 2003 Mar 18. J Biol Chem. 2003. PMID: 12646575
-
Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities.J Virol. 2002 Aug;76(16):8161-8. doi: 10.1128/jvi.76.16.8161-8168.2002. J Virol. 2002. PMID: 12134021 Free PMC article.
-
Human Cytomegalovirus US28: a functionally selective chemokine binding receptor.Infect Disord Drug Targets. 2009 Nov;9(5):548-56. doi: 10.2174/187152609789105696. Infect Disord Drug Targets. 2009. PMID: 19594424 Free PMC article. Review.
-
Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28.J Biol Chem. 2001 Jan 12;276(2):1133-7. doi: 10.1074/jbc.M008965200. J Biol Chem. 2001. PMID: 11050102
-
G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors.Pharmacology. 2010;86(1):22-9. doi: 10.1159/000314161. Epub 2010 Jun 26. Pharmacology. 2010. PMID: 20693822 Free PMC article. Review.
Cited by
-
Exosomal release of the virus-encoded chemokine receptor US28 contributes to chemokine scavenging.iScience. 2023 Jul 18;26(8):107412. doi: 10.1016/j.isci.2023.107412. eCollection 2023 Aug 18. iScience. 2023. PMID: 37575190 Free PMC article.
-
Heteromerization of human cytomegalovirus encoded chemokine receptors.Biochem Pharmacol. 2011 Sep 15;82(6):610-9. doi: 10.1016/j.bcp.2011.06.009. Epub 2011 Jun 13. Biochem Pharmacol. 2011. PMID: 21684267 Free PMC article.
-
US28, a Virally-Encoded GPCR as an Antiviral Target for Human Cytomegalovirus Infection.Biomol Ther (Seoul). 2017 Jan 1;25(1):69-79. doi: 10.4062/biomolther.2016.208. Biomol Ther (Seoul). 2017. PMID: 28035083 Free PMC article. Review.
-
Gα13 mediates human cytomegalovirus-encoded chemokine receptor US28-induced cell death in melanoma.Int J Cancer. 2015 Sep 15;137(6):1503-8. doi: 10.1002/ijc.29506. Epub 2015 Mar 16. Int J Cancer. 2015. PMID: 25754407 Free PMC article.
-
Identification of common mechanisms by which human and mouse cytomegalovirus seven-transmembrane receptor homologues contribute to in vivo phenotypes in a mouse model.J Virol. 2013 Apr;87(7):4112-7. doi: 10.1128/JVI.03406-12. Epub 2013 Jan 23. J Virol. 2013. PMID: 23345521 Free PMC article.
References
-
- Michelson S. Consequences of human cytomegalovirus mimicry. Hum Immunol. 2004;65:465–475. - PubMed
-
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. - PubMed
-
- Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

