Drug-free macromolecular therapeutics: induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface
- PMID: 20101660
- PMCID: PMC2998410
- DOI: 10.1002/anie.200906232
Drug-free macromolecular therapeutics: induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface
Abstract
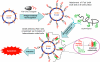
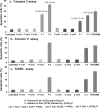
A new paradigm was designed for apoptosis induction mediated by the biorecognition of coiled-coil motifs at the Raji B cell surface. The heterodimerization of complementary peptides, one bound to Fab’ antibody fragment, the other as grafts to HPMA copolymer, results in crosslinking of CD20 target antigens, and consequently, initiation of apoptosis.
Figures


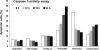
Similar articles
-
Human Serum Albumin-Based Drug-Free Macromolecular Therapeutics: Apoptosis Induction by Coiled-Coil-Mediated Cross-Linking of CD20 Antigens on Lymphoma B Cell Surface.Macromol Biosci. 2018 Nov;18(11):e1800224. doi: 10.1002/mabi.201800224. Epub 2018 Sep 27. Macromol Biosci. 2018. PMID: 30259654 Free PMC article.
-
Tuning assembly size in Peptide-based supramolecular polymers by modulation of subunit association affinity.Biomacromolecules. 2014 Apr 14;15(4):1436-42. doi: 10.1021/bm5000423. Epub 2014 Mar 17. Biomacromolecules. 2014. PMID: 24598042
-
Development of a series of cross-linking agents that effectively stabilize alpha-helical structures in various short peptides.Chemistry. 2008;14(3):857-63. doi: 10.1002/chem.200700843. Chemistry. 2008. PMID: 17969217
-
Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking.Adv Protein Chem. 2003;63:243-90. doi: 10.1016/s0065-3233(03)63010-x. Adv Protein Chem. 2003. PMID: 12629973 Review. No abstract available.
-
Design of smart HPMA copolymer-based nanomedicines.J Control Release. 2016 Oct 28;240:9-23. doi: 10.1016/j.jconrel.2015.10.003. Epub 2015 Oct 3. J Control Release. 2016. PMID: 26437260 Free PMC article. Review.
Cited by
-
Coiled-coil networking shapes cell molecular machinery.Mol Biol Cell. 2012 Oct;23(19):3911-22. doi: 10.1091/mbc.E12-05-0396. Epub 2012 Aug 8. Mol Biol Cell. 2012. PMID: 22875988 Free PMC article.
-
Smart self-assembled hybrid hydrogel biomaterials.Angew Chem Int Ed Engl. 2012 Jul 23;51(30):7396-417. doi: 10.1002/anie.201201040. Angew Chem Int Ed Engl. 2012. PMID: 22806947 Free PMC article. Review.
-
Critical evaluation of in silico methods for prediction of coiled-coil domains in proteins.Brief Bioinform. 2016 Mar;17(2):270-82. doi: 10.1093/bib/bbv047. Epub 2015 Jul 15. Brief Bioinform. 2016. PMID: 26177815 Free PMC article. Review.
-
Immunogenicity of coiled-coil based drug-free macromolecular therapeutics.Biomaterials. 2014 Jul;35(22):5886-96. doi: 10.1016/j.biomaterials.2014.03.063. Epub 2014 Apr 22. Biomaterials. 2014. PMID: 24767787 Free PMC article.
-
Drug-Free Macromolecular Therapeutics Induce Apoptosis via Calcium Influx and Mitochondrial Signaling Pathway.Macromol Biosci. 2018 Jan;18(1):10.1002/mabi.201700196. doi: 10.1002/mabi.201700196. Epub 2017 Aug 14. Macromol Biosci. 2018. PMID: 28805013 Free PMC article.
References
-
- Wang C, Stewart RJ, Kopeček J. Nature. 1999;397:417. - PubMed
-
- Kosmas, Stamatopoulos K, Stavroyianni N, Tsavaris N, Papadaki T. Leukemia. 2002;16:2004. - PubMed
- Deans JP, Li H, Polyak MJ. Immunology. 2002;107:176. - PMC - PubMed
- Golay JT, Clark EA, Beverley PC. J. Immunol. 1985;135:3795. - PubMed
- Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. J. Cell Biol. 1993;121:1121. - PMC - PubMed
- Press OW, Farr AG, Borroz KI, Anderson SK, Martin PJ. Cancer Res. 1989;49:4906. - PubMed
- Press OW, Appelbaum F, Ledbetter JA, Martin PJ, Zarling J, Kidd P, Thomas ED. Blood. 1987;69:584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

