Reduced expression of the Ca(2+) transporter protein PMCA2 slows Ca(2+) dynamics in mouse cerebellar Purkinje neurones and alters the precision of motor coordination
- PMID: 20083513
- PMCID: PMC2849958
- DOI: 10.1113/jphysiol.2009.182196
Reduced expression of the Ca(2+) transporter protein PMCA2 slows Ca(2+) dynamics in mouse cerebellar Purkinje neurones and alters the precision of motor coordination
Abstract
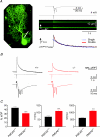
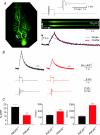
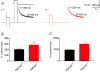
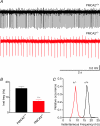
Cerebellar Purkinje neurones (PNs) express high levels of the plasma membrane calcium ATPase, PMCA2, a transporter protein critical for the clearance of calcium from excitable cells. Genetic deletion of one PMCA2 encoding gene in heterozygous PMCA2 knock-out (PMCA2(+/-) mice enabled us to determine how PMCA2 influences PN calcium regulation without the complication of the severe morphological changes associated with complete PMCA2 knock-out (PMCA2(-/-) in these cells. The PMCA2(+/-) cerebellum expressed half the normal levels of PMCA2 and this nearly doubled the time taken for PN dendritic calcium transients to recover (mean fast and slow recovery times increased from 70 ms to 110 ms and from 600 ms to 1100 ms). The slower calcium recovery had distinct consequences for PMCA2(+/-) PN physiology. The PNs exhibited weaker climbing fibre responses, prolonged outward Ca(2+)-dependent K(+) current (mean fast and slow recovery times increased from 136 ms to 192 ms and from 595 ms to 1423 ms) and a slower mean frequency of action potential firing (7.4 Hz compared with 15.8 Hz). Our findings were consistent with prolonged calcium accumulation in the cytosol of PMCA2(+/-) Purkinje neurones. Although PMCA2(+/-) mice exhibited outwardly normal behaviour and little change in their gait pattern, when challenged to run on a narrow beam they exhibited clear deficits in hindlimb coordination. Training improved the motor performance of both PMCA2(+/-) and wild-type mice, although PMCA2(+/-) mice were always impaired. We conclude that reduced calcium clearance perturbs calcium dynamics in PN dendrites and that this is sufficient to disrupt the accuracy of cerebellar processing and motor coordination.
Figures


Similar articles
-
Purkinje cell dysfunction and delayed death in plasma membrane calcium ATPase 2-heterozygous mice.Mol Cell Neurosci. 2012 Aug;51(1-2):22-31. doi: 10.1016/j.mcn.2012.07.001. Epub 2012 Jul 10. Mol Cell Neurosci. 2012. PMID: 22789621 Free PMC article.
-
The role of the calcium transporter protein plasma membrane calcium ATPase PMCA2 in cerebellar Purkinje neuron function.Funct Neurol. 2010 Jul-Sep;25(3):153-8. Funct Neurol. 2010. PMID: 21232211
-
Molecular alterations in the cerebellum of the plasma membrane calcium ATPase 2 (PMCA2)-null mouse indicate abnormalities in Purkinje neurons.Mol Cell Neurosci. 2007 Feb;34(2):178-88. doi: 10.1016/j.mcn.2006.10.010. Epub 2006 Dec 5. Mol Cell Neurosci. 2007. PMID: 17150372 Free PMC article.
-
Survival strategies for mouse cerebellar Purkinje neurons lacking PMCA2.Neurosci Lett. 2018 Jan 10;663:25-28. doi: 10.1016/j.neulet.2017.09.045. Neurosci Lett. 2018. PMID: 29452612 Review.
-
The plasma membrane calcium pump in the hearing process: physiology and pathology.Sci China Life Sci. 2011 Aug;54(8):686-90. doi: 10.1007/s11427-011-4200-z. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786191 Review.
Cited by
-
Calcium Dyshomeostasis Alters CCL5 Signaling in Differentiated PC12 Cells.Biomed Res Int. 2019 Mar 26;2019:9616248. doi: 10.1155/2019/9616248. eCollection 2019. Biomed Res Int. 2019. PMID: 31032369 Free PMC article.
-
A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2.Elife. 2017 May 18;6:e26377. doi: 10.7554/eLife.26377. Elife. 2017. PMID: 28518055 Free PMC article.
-
Purkinje cell dysfunction and delayed death in plasma membrane calcium ATPase 2-heterozygous mice.Mol Cell Neurosci. 2012 Aug;51(1-2):22-31. doi: 10.1016/j.mcn.2012.07.001. Epub 2012 Jul 10. Mol Cell Neurosci. 2012. PMID: 22789621 Free PMC article.
-
Functional integration of calcium regulatory mechanisms at Purkinje neuron synapses.Cerebellum. 2012 Sep;11(3):640-50. doi: 10.1007/s12311-010-0185-6. Cerebellum. 2012. PMID: 20596808 Free PMC article. Review.
-
Cellular and circuit mechanisms underlying spinocerebellar ataxias.J Physiol. 2016 Aug 15;594(16):4653-60. doi: 10.1113/JP271897. Epub 2016 Jun 12. J Physiol. 2016. PMID: 27198167 Free PMC article. Review.
References
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Berrocal M, Marcos D, Sepúlveda MR, Pérez M, Avila J, Mata AM. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer's disease. FASEB J. 2009;23:1826–1834. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

