Mammalian sirtuins: biological insights and disease relevance
- PMID: 20078221
- PMCID: PMC2866163
- DOI: 10.1146/annurev.pathol.4.110807.092250
Mammalian sirtuins: biological insights and disease relevance
Abstract
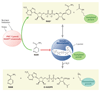
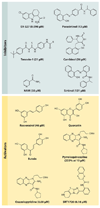
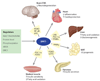
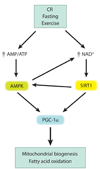
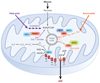
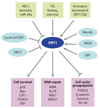
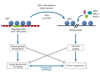
Aging is accompanied by a decline in the healthy function of multiple organ systems, leading to increased incidence and mortality from diseases such as type II diabetes mellitus, neurodegenerative diseases, cancer, and cardiovascular disease. Historically, researchers have focused on investigating individual pathways in isolated organs as a strategy to identify the root cause of a disease, with hopes of designing better drugs. Studies of aging in yeast led to the discovery of a family of conserved enzymes known as the sirtuins, which affect multiple pathways that increase the life span and the overall health of organisms. Since the discovery of the first known mammalian sirtuin, SIRT1, 10 years ago, there have been major advances in our understanding of the enzymology of sirtuins, their regulation, and their ability to broadly improve mammalian physiology and health span. This review summarizes and discusses the advances of the past decade and the challenges that will confront the field in the coming years.
Figures
Similar articles
-
The sirtuin family's role in aging and age-associated pathologies.J Clin Invest. 2013 Mar;123(3):973-9. doi: 10.1172/JCI64094. Epub 2013 Mar 1. J Clin Invest. 2013. PMID: 23454760 Free PMC article. Review.
-
Sirtuins in aging and age-related disease.Cell. 2006 Jul 28;126(2):257-68. doi: 10.1016/j.cell.2006.07.002. Cell. 2006. PMID: 16873059 Review.
-
Recent progress in the biology and physiology of sirtuins.Nature. 2009 Jul 30;460(7255):587-91. doi: 10.1038/nature08197. Nature. 2009. PMID: 19641587 Free PMC article. Review.
-
SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology.Physiology (Bethesda). 2006 Dec;21:404-10. doi: 10.1152/physiol.00031.2006. Physiology (Bethesda). 2006. PMID: 17119153 Review.
-
Sirtuins and beta-cell function.Diabetes Obes Metab. 2007 Nov;9 Suppl 2:23-7. doi: 10.1111/j.1463-1326.2007.00769.x. Diabetes Obes Metab. 2007. PMID: 17919175
Cited by
-
Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT.Aging Cell. 2013 Dec;12(6):1062-72. doi: 10.1111/acel.12135. Epub 2013 Aug 11. Aging Cell. 2013. PMID: 23834033 Free PMC article.
-
Role of histone modification in the occurrence and development of osteoporosis.Front Endocrinol (Lausanne). 2022 Aug 26;13:964103. doi: 10.3389/fendo.2022.964103. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093077 Free PMC article. Review.
-
The NAD(+)-dependent protein deacetylase activity of SIRT1 is regulated by its oligomeric status.Sci Rep. 2012;2:640. doi: 10.1038/srep00640. Epub 2012 Sep 7. Sci Rep. 2012. PMID: 22962634 Free PMC article.
-
Advance in Iron Metabolism, Oxidative Stress and Cellular Dysfunction in Experimental and Human Kidney Diseases.Antioxidants (Basel). 2024 May 27;13(6):659. doi: 10.3390/antiox13060659. Antioxidants (Basel). 2024. PMID: 38929098 Free PMC article. Review.
-
Decoding the rosetta stone of mitonuclear communication.Pharmacol Res. 2020 Nov;161:105161. doi: 10.1016/j.phrs.2020.105161. Epub 2020 Aug 23. Pharmacol Res. 2020. PMID: 32846213 Free PMC article. Review.
References
-
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell. 1997;91:1033–1042. - PubMed
-
- Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci. Am. 2006;294:48–57. - PubMed
-
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. - PubMed
-
- Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem. Sci. 2007;32:555–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG027916-030003/AG/NIA NIH HHS/United States
- P01 AG027916-020003/AG/NIA NIH HHS/United States
- P01 AG027916-010003/AG/NIA NIH HHS/United States
- P01 AG027916-04S20003/AG/NIA NIH HHS/United States
- R01 AG028730/AG/NIA NIH HHS/United States
- R01 AG019719-07/AG/NIA NIH HHS/United States
- R01 AG028730-02/AG/NIA NIH HHS/United States
- R01 AG028730-01A1/AG/NIA NIH HHS/United States
- R01 AG019719/AG/NIA NIH HHS/United States
- R01 AG019719-06A1/AG/NIA NIH HHS/United States
- R01 AG028730-03/AG/NIA NIH HHS/United States
- P01 AG027916/AG/NIA NIH HHS/United States
- P01 AG027916-04S10003/AG/NIA NIH HHS/United States
- R01 AG032375/AG/NIA NIH HHS/United States
- P01 AG027916-040003/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

