Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10
- PMID: 20071504
- PMCID: PMC2822595
- DOI: 10.1084/jem.20091959
Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10
Abstract
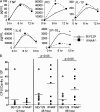
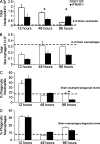
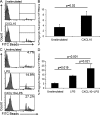
Type I interferon (IFN) alpha/beta is critical for host defense. During endotoxicosis or highly lethal bacterial infections where systemic inflammation predominates, mice deficient in IFN-alpha/beta receptor (IFNAR) display decreased systemic inflammation and improved outcome. However, human sepsis mortality often occurs during a prolonged period of immunosuppression and not from exaggerated inflammation. We used a low lethality cecal ligation and puncture (CLP) model of sepsis to determine the role of type I IFNs in host defense during sepsis. Despite increased endotoxin resistance, IFNAR(-/-) and chimeric mice lacking IFNAR in hematopoietic cells display increased mortality to CLP. This was not associated with an altered early systemic inflammatory response, except for decreased CXCL10 production. IFNAR(-/-) mice display persistently elevated peritoneal bacterial counts compared with wild-type mice, reduced peritoneal neutrophil recruitment, and recruitment of neutrophils with poor phagocytic function despite normal to enhanced adaptive immune function during sepsis. Importantly, CXCL10 treatment of IFNAR(-/-) mice improves survival and decreases peritoneal bacterial loads, and CXCL10 increases mouse and human neutrophil phagocytosis. Using a low lethality sepsis model, we identify a critical role of type I IFN-dependent CXCL10 in host defense during polymicrobial sepsis by increasing neutrophil recruitment and function.
Figures


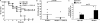
Similar articles
-
IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture.J Immunol. 2006 Sep 1;177(5):3218-24. doi: 10.4049/jimmunol.177.5.3218. J Immunol. 2006. PMID: 16920961
-
Type I IFN modulates host defense and late hyperinflammation in septic peritonitis.J Immunol. 2006 Oct 15;177(8):5623-30. doi: 10.4049/jimmunol.177.8.5623. J Immunol. 2006. PMID: 17015750
-
Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival.J Immunol. 2010 Dec 1;185(11):6930-8. doi: 10.4049/jimmunol.1002300. Epub 2010 Nov 1. J Immunol. 2010. PMID: 21041722 Free PMC article.
-
Pharmacological inhibition of type I interferon signaling protects mice against lethal sepsis.J Infect Dis. 2014 Mar;209(6):960-70. doi: 10.1093/infdis/jit600. Epub 2013 Nov 11. J Infect Dis. 2014. PMID: 24218508
-
Phagocytosis-Inflammation Crosstalk in Sepsis: New Avenues for Therapeutic Intervention.Shock. 2020 Nov;54(5):606-614. doi: 10.1097/SHK.0000000000001541. Shock. 2020. PMID: 32516170 Free PMC article. Review.
Cited by
-
Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): a review of definitions, potential therapies, and research priorities.Br J Anaesth. 2024 Mar;132(3):507-518. doi: 10.1016/j.bja.2023.11.052. Epub 2024 Jan 4. Br J Anaesth. 2024. PMID: 38177003 Free PMC article. Review.
-
Cyclic GMP-AMP synthase contributes to epithelial homeostasis in intestinal inflammation via Beclin-1-mediated autophagy.FASEB J. 2022 May;36(5):e22282. doi: 10.1096/fj.202200138R. FASEB J. 2022. PMID: 35344224 Free PMC article.
-
IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation.Nat Commun. 2019 Aug 2;10(1):3471. doi: 10.1038/s41467-019-10903-9. Nat Commun. 2019. PMID: 31375662 Free PMC article.
-
Nonhematopoietic β-Arrestin-1 inhibits inflammation in a murine model of polymicrobial sepsis.Am J Pathol. 2014 Aug;184(8):2297-309. doi: 10.1016/j.ajpath.2014.05.002. Epub 2014 Jun 17. Am J Pathol. 2014. PMID: 24946011 Free PMC article.
-
IFNα enhances the production of IL-6 by human neutrophils activated via TLR8.Sci Rep. 2016 Jan 21;6:19674. doi: 10.1038/srep19674. Sci Rep. 2016. PMID: 26790609 Free PMC article.
References
-
- Delano M.J., Scumpia P.O., Weinstein J.S., Coco D., Nagaraj S., Kelly-Scumpia K.M., O’Malley K.A., Wynn J.L., Antonenko S., Al-Quran S.Z., et al. 2007. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463–1474 10.1084/jem.20062602 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

