Toll-like receptor 6 drives interleukin-17A expression during experimental hypersensitivity pneumonitis
- PMID: 20070409
- PMCID: PMC2855800
- DOI: 10.1111/j.1365-2567.2009.03219.x
Toll-like receptor 6 drives interleukin-17A expression during experimental hypersensitivity pneumonitis
Abstract
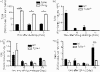
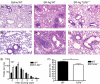
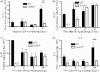
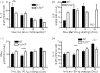
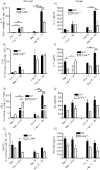
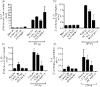
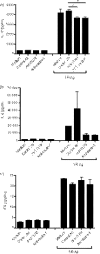
Hypersensitivity pneumonitis (HP) is a T-cell-driven disease that is histologically characterized by diffuse mononuclear cell infiltrates and loosely formed granulomas in the lungs. We have previously reported that interleukin-17A (IL-17A) contributes to the development of experimental HP, and that the pattern recognition receptor Toll-like receptor 6 (TLR6) might be a factor in the initiation of this response. Using a well-established murine model of Saccharopolyspora rectivirgula-induced HP, we investigated the role of TLR6 in the immunopathogenesis of this disease. In the absence of TLR6 signalling, mice that received multiple challenges with S. rectivirgula-antigen (SR-Ag) had significantly less lung inflammation compared with C57BL/6 mice (wild-type; WT) similarly challenged with SR-Ag. Flow cytometric analysis of whole lung samples from SR-Ag-challenged mice showed that TLR6(-/-) mice had a decreased CD4(+) : CD8(+) T-cell ratio compared with WT mice. Cytokine analysis at various days after the final SR-Ag challenge revealed that whole lungs from TLR6(-/-) mice contained significantly less IL-17A than lungs from WT mice with HP. The IL-17A-driving cytokines IL-21 and IL-23 were also expressed at lower levels in SR-Ag-challenged TLR6(-/-) mice, when compared with SR-Ag-challenged WT mice. Other pro-inflammatory cytokines, namely interferon-gamma and RANTES, were also found to be regulated by TLR6 signalling. Anti-TLR6 neutralizing antibody treatment of dispersed lung cells significantly impaired SR-Ag-induced IL-17A and IL-6 generation. Together, these results indicate that TLR6 plays a pivotal role in the development and severity of HP via its role in IL-17A production.
Figures

Similar articles
-
Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response.Am J Physiol Lung Cell Mol Physiol. 2016 Mar 1;310(5):L393-402. doi: 10.1152/ajplung.00305.2015. Epub 2015 Dec 30. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26719148 Free PMC article.
-
Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis.Am J Respir Crit Care Med. 2009 Apr 15;179(8):705-16. doi: 10.1164/rccm.200811-1700OC. Epub 2009 Jan 16. Am J Respir Crit Care Med. 2009. PMID: 19151189
-
Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis.J Immunol. 2009 Jan 1;182(1):657-65. J Immunol. 2009. PMID: 19109199 Free PMC article.
-
IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis.J Immunol. 2009 May 15;182(10):6540-9. doi: 10.4049/jimmunol.0900013. J Immunol. 2009. PMID: 19414809 Free PMC article.
-
Protein kinase D1 in myeloid lineage cells contributes to the accumulation of CXCR3+CCR6+ nonconventional Th1 cells in the lungs and potentiates hypersensitivity pneumonitis caused by S. rectivirgula.Front Immunol. 2024 Oct 11;15:1403155. doi: 10.3389/fimmu.2024.1403155. eCollection 2024. Front Immunol. 2024. PMID: 39464896 Free PMC article.
Cited by
-
Biomarkers associated with immune-related adverse events induced by immune checkpoint inhibitors.World J Clin Oncol. 2024 Aug 24;15(8):1002-1020. doi: 10.5306/wjco.v15.i8.1002. World J Clin Oncol. 2024. PMID: 39193157 Free PMC article. Review.
-
Management of hypersensivity pneumonitis.Clin Transl Allergy. 2013 Feb 4;3(1):5. doi: 10.1186/2045-7022-3-5. Clin Transl Allergy. 2013. PMID: 23374544 Free PMC article.
-
Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response.Am J Physiol Lung Cell Mol Physiol. 2016 Mar 1;310(5):L393-402. doi: 10.1152/ajplung.00305.2015. Epub 2015 Dec 30. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26719148 Free PMC article.
-
Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors.Cancer Immunol Immunother. 2021 Aug;70(8):2209-2221. doi: 10.1007/s00262-021-02855-1. Epub 2021 Jan 22. Cancer Immunol Immunother. 2021. PMID: 33481042 Free PMC article.
-
Diagnosis of Fibrotic Hypersensitivity Pneumonitis: Is There a Role for Biomarkers?Life (Basel). 2023 Feb 17;13(2):565. doi: 10.3390/life13020565. Life (Basel). 2023. PMID: 36836922 Free PMC article. Review.
References
-
- Barrios RJ. Hypersensitivity pneumonitis: histopathology. Arch Pathol Lab Med. 2008;132:199–203. - PubMed
-
- Takemura T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Pathology of hypersensitivity pneumonitis. Curr Opin Pulm Med. 2008;14:440–54. - PubMed
-
- Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30:201–8. - PubMed
-
- Madison JM. Hypersensitivity pneumonitis: clinical perspectives. Arch Pathol Lab Med. 2008;132:195–8. - PubMed
-
- Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

