Hydrogen peroxide-sensitive cysteines in the Sty1 MAPK regulate the transcriptional response to oxidative stress
- PMID: 20061379
- PMCID: PMC2844198
- DOI: 10.1074/jbc.M109.040840
Hydrogen peroxide-sensitive cysteines in the Sty1 MAPK regulate the transcriptional response to oxidative stress
Abstract
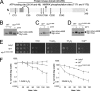
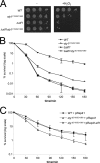
MAPK are activated by and orchestrate responses to multiple, diverse stimuli. Although these responses involve the increased phosphorylation of substrate effector proteins, e.g. transcription factors, the mechanisms by which responses are tailored to particular stimuli are unclear. In the fission yeast Schizosaccharomyces pombe, the Sty1 MAPK is crucial for changes in gene expression that allow adaptation to many forms of environmental stress. Here, we have identified two cysteine residues in Sty1, Cys-153 and Cys-158, that are important for hydrogen peroxide-induced gene expression and oxidative stress resistance but not for other functions of Sty1. Many Sty1-dependent changes in gene expression are mediated by the Atf1 transcription factor. In response to stress, Sty1 increases Atf1 levels by (i) promoting increases in atf1 mRNA and by (ii) directly phosphorylating and stabilizing Atf1 protein. Although dispensable for phosphorylation and stabilization of Atf1 protein, we find that both Cys-153 and Cys-158 are required for increases in atf1 mRNA levels and Atf1-dependent gene expression in response to hydrogen peroxide but not osmotic stress. Indeed, our data indicate that oxidation of Sty1, by formation of a disulfide bond between Cys-153 and Cys-158, is important for maintaining atf1 mRNA stability at high concentrations of hydrogen peroxide. Together, these data reveal that redox regulation of cysteine thiols in Sty1 is involved in a stress-specific mechanism regulating transcriptional responses to oxidative stress. Intriguingly, the conservation of these cysteine residues in other MAPK raises the possibility that similar mechanisms may ensure appropriate responses to hydrogen peroxide in other eukaryotes.
Figures


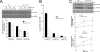


Similar articles
-
Deciphering the role of the signal- and Sty1 kinase-dependent phosphorylation of the stress-responsive transcription factor Atf1 on gene activation.J Biol Chem. 2017 Aug 18;292(33):13635-13644. doi: 10.1074/jbc.M117.794339. Epub 2017 Jun 26. J Biol Chem. 2017. PMID: 28652406 Free PMC article.
-
Regulation of Schizosaccharomyces pombe Atf1 protein levels by Sty1-mediated phosphorylation and heterodimerization with Pcr1.J Biol Chem. 2007 Feb 23;282(8):5160-70. doi: 10.1074/jbc.M608526200. Epub 2006 Dec 20. J Biol Chem. 2007. PMID: 17182615
-
Transcription factors Atf1 and Sty1 promote stress tolerance under nitrosative stress in Schizosaccharomyces pombe.Microbiol Res. 2018 Jan;206:82-90. doi: 10.1016/j.micres.2017.10.002. Epub 2017 Oct 12. Microbiol Res. 2018. PMID: 29146263
-
Phospho-mimicking Atf1 mutants bypass the transcription activating function of the MAP kinase Sty1 of fission yeast.Curr Genet. 2018 Feb;64(1):97-102. doi: 10.1007/s00294-017-0730-7. Epub 2017 Aug 10. Curr Genet. 2018. PMID: 28799013 Review.
-
Nuclear roles and regulation of chromatin structure by the stress-dependent MAP kinase Sty1 of Schizosaccharomyces pombe.Mol Microbiol. 2011 Nov;82(3):542-54. doi: 10.1111/j.1365-2958.2011.07851.x. Epub 2011 Oct 13. Mol Microbiol. 2011. PMID: 21992435 Review.
Cited by
-
Thiol-based direct threat sensing by the stress-activated protein kinase Hog1.Sci Signal. 2019 Nov 26;12(609):eaaw4956. doi: 10.1126/scisignal.aaw4956. Sci Signal. 2019. PMID: 31772124 Free PMC article.
-
Communication between Cyclin-dependent kinase Cdc2 and the Wis1-Spc1 MAPK pathway determines mitotic timing in Schizosaccharomyces pombe.Biol Open. 2020 Jul 21;9(7):bio053322. doi: 10.1242/bio.053322. Biol Open. 2020. PMID: 32554481 Free PMC article.
-
Guidelines and recommendations on yeast cell death nomenclature.Microb Cell. 2018 Jan 1;5(1):4-31. doi: 10.15698/mic2018.01.607. Microb Cell. 2018. PMID: 29354647 Free PMC article. Review.
-
Fission yeast Cdc14-like phosphatase Flp1/Clp1 modulates the transcriptional response to oxidative stress.Sci Rep. 2023 Sep 6;13(1):14677. doi: 10.1038/s41598-023-41869-w. Sci Rep. 2023. PMID: 37674027 Free PMC article.
-
Cysteine-based redox switches in enzymes.Antioxid Redox Signal. 2011 Mar 15;14(6):1065-77. doi: 10.1089/ars.2010.3376. Epub 2010 Sep 17. Antioxid Redox Signal. 2011. PMID: 20799881 Free PMC article. Review.
References
-
- Wagner E. F., Nebreda A. R. (2009) Nat. Rev. Cancer 9, 537–549 - PubMed
-
- Dong C., Davis R. J., Flavell R. A. (2002) Annu. Rev. Immunol. 20, 55–72 - PubMed
-
- Kim D. H., Feinbaum R., Alloing G., Emerson F. E., Garsin D. A., Inoue H., Tanaka-Hino M., Hisamoto N., Matsumoto K., Tan M. W., Ausubel F. M. (2002) Science 297, 623–626 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

