ATR activation and replication fork restart are defective in FANCM-deficient cells
- PMID: 20057355
- PMCID: PMC2829160
- DOI: 10.1038/emboj.2009.385
ATR activation and replication fork restart are defective in FANCM-deficient cells
Abstract
Fanconi anaemia is a chromosomal instability disorder associated with cancer predisposition and bone marrow failure. Among the 13 identified FA gene products only one, the DNA translocase FANCM, has homologues in lower organisms, suggesting a conserved function in DNA metabolism. However, a precise role for FANCM in DNA repair remains elusive. Here, we show a novel function for FANCM that is distinct from its role in the FA pathway: promoting replication fork restart and simultaneously limiting the accumulation of RPA-ssDNA. We show that in DT40 cells this process is controlled by ATR and PLK1, and that in the absence of FANCM, stalled replication forks are unable to resume DNA synthesis and genome duplication is ensured by excess origin firing. Unexpectedly, we also uncover an early role for FANCM in ATR-mediated checkpoint signalling by promoting chromatin retention of TopBP1. Failure to retain TopBP1 on chromatin impacts on the ability of ATR to phosphorylate downstream molecular targets, including Chk1 and SMC1. Our data therefore indicate a fundamental role for FANCM in the maintenance of genome integrity during S phase.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
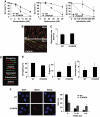
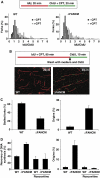
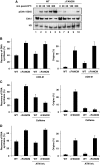

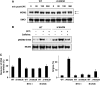
Comment in
-
FANCM: fork pause, rewind and play.EMBO J. 2010 Feb 17;29(4):703-5. doi: 10.1038/emboj.2009.415. EMBO J. 2010. PMID: 20160754 Free PMC article.
Similar articles
-
The DNA translocase activity of FANCM protects stalled replication forks.Hum Mol Genet. 2012 May 1;21(9):2005-16. doi: 10.1093/hmg/dds013. Epub 2012 Jan 24. Hum Mol Genet. 2012. PMID: 22279085
-
The Fanconi anemia protein FANCM is controlled by FANCD2 and the ATR/ATM pathways.J Biol Chem. 2009 Sep 18;284(38):25560-8. doi: 10.1074/jbc.M109.007690. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19633289 Free PMC article.
-
FANCM and FAAP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex.Mol Cell. 2008 Nov 7;32(3):313-24. doi: 10.1016/j.molcel.2008.10.014. Mol Cell. 2008. PMID: 18995830
-
The ATR pathway: fine-tuning the fork.DNA Repair (Amst). 2007 Jul 1;6(7):953-66. doi: 10.1016/j.dnarep.2007.02.015. Epub 2007 May 24. DNA Repair (Amst). 2007. PMID: 17531546 Review.
-
FANCM-FAAP24 and HCLK2: roles in ATR signalling and the Fanconi anemia pathway.Cell Cycle. 2009 Apr 15;8(8):1133-7. doi: 10.4161/cc.8.8.8204. Epub 2009 Apr 16. Cell Cycle. 2009. PMID: 19282663 Review.
Cited by
-
A novel interplay between the Fanconi anemia core complex and ATR-ATRIP kinase during DNA cross-link repair.Nucleic Acids Res. 2013 Aug;41(14):6930-41. doi: 10.1093/nar/gkt467. Epub 2013 May 30. Nucleic Acids Res. 2013. PMID: 23723247 Free PMC article.
-
FANCM and FAAP24 maintain genome stability via cooperative as well as unique functions.Mol Cell. 2013 Mar 7;49(5):997-1009. doi: 10.1016/j.molcel.2012.12.010. Epub 2013 Jan 17. Mol Cell. 2013. PMID: 23333308 Free PMC article.
-
FANCM, BRCA1, and BLM cooperatively resolve the replication stress at the ALT telomeres.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E5940-E5949. doi: 10.1073/pnas.1708065114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673972 Free PMC article.
-
miR146a-mediated targeting of FANCM during inflammation compromises genome integrity.Oncotarget. 2016 Jul 19;7(29):45976-45994. doi: 10.18632/oncotarget.10275. Oncotarget. 2016. PMID: 27351285 Free PMC article.
-
Levels of the E2 interacting protein TopBP1 modulate papillomavirus maintenance stage replication.Virology. 2015 Apr;478:129-35. doi: 10.1016/j.virol.2015.01.011. Epub 2015 Feb 7. Virology. 2015. PMID: 25666521 Free PMC article.
References
-
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 - PubMed
-
- Bakker ST, van de Vrugt HJ, Rooimans MA, Oostra AB, Steltenpool J, Delzenne-Goette E, van der Wal A, van der Valk M, Joenje H, Te Riele H, de Winter JP (2009) Fancm-deficient mice reveal unique features of Fanconi anemia complementation group M. Hum Mol Genet 18: 3484–3495 - PubMed
-
- Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetei AR, Laghmani el H, Joenje H, McDonald N, de Winter JP, Wang W, West SC (2007) Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol Cell 25: 331–343 - PubMed
-
- Collis SJ, Barber LJ, Clark AJ, Martin JS, Ward JD, Boulton SJ (2007) HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat Cell Biol 9: 391–401 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

