Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway
- PMID: 20052412
- PMCID: PMC2797399
- DOI: 10.1371/journal.pone.0008576
Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway
Abstract
Background: We hypothesized that genetic modification of mesenchymal stem cells (MSCs) with Sonic Hedgehog (Shh) transgene, a morphogen during embryonic development and embryonic and adult stem cell growth, improved their survival and angiogenic potential in the ischemic heart via iNOS/netrin/PKC pathway.
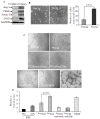
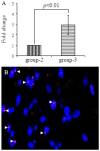
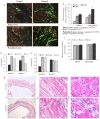
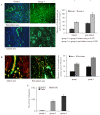
Methods/principal findings: MSCs from young Fisher-344 rat bone marrow were purified and transfected with pCMV Shh plasmid ((Shh)MSCs). Immunofluorescence, RT-PCR and Western blotting showed higher expression of Shh in (Shh)MSCs which also led to increased expression of angiogenic and pro-survival growth factors in (Shh)MSCs. Significantly improved migration and tube formation was seen in (Shh)MSCs as compared to empty vector transfected MSCs ((Emp)MSCs). Significant upregulation of netrin-1 and iNOS was observed in (Shh)MSCs in PI3K independent but PKC dependent manner. For in vivo studies, acute myocardial infarction model was developed in Fisher-344 rats. The animals were grouped to receive 70 microl basal DMEM without cells (group-1) or containing 1x10(6) (Emp)MSCs (group-2) and (Shh)MSCs (group-3). Group-4 received recombinant netrin-1 protein injection into the infarcted heart. FISH and sry-quantification revealed improved survival of (Shh)MSCs post engraftment. Histological studies combined with fluorescent microspheres showed increased density of functionally competent blood vessels in group-3 and group-4. Echocardiography showed significantly preserved heart function indices post engraftment with (Shh)MSCs in group-3 animals.
Conclusions/significance: Reprogramming of stem cells with Shh maximizes their survival and angiogenic potential in the heart via iNOS/netrin-1/PKC signaling.
Conflict of interest statement
Figures



Similar articles
-
Netrin-1 ameliorates myocardial infarction-induced myocardial injury: mechanisms of action in rats and diabetic mice.Hum Gene Ther. 2014 Sep;25(9):787-97. doi: 10.1089/hum.2014.021. Epub 2014 Jun 18. Hum Gene Ther. 2014. PMID: 24827071 Free PMC article.
-
Cytoprotective and proangiogenic activity of ex-vivo netrin-1 transgene overexpression protects the heart against ischemia/reperfusion injury.Stem Cells Dev. 2012 Jul 1;21(10):1769-78. doi: 10.1089/scd.2011.0475. Epub 2011 Nov 11. Stem Cells Dev. 2012. PMID: 21936706 Free PMC article.
-
Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells.J Mol Cell Cardiol. 2010 Sep;49(3):490-8. doi: 10.1016/j.yjmcc.2010.05.003. Epub 2010 May 15. J Mol Cell Cardiol. 2010. PMID: 20478312 Free PMC article.
-
Netrin-1 role in angiogenesis: to be or not to be a pro-angiogenic factor?Cell Cycle. 2010 Apr 15;9(8):1466-71. doi: 10.4161/cc.9.8.11197. Epub 2010 Apr 15. Cell Cycle. 2010. PMID: 20372055 Review.
-
The morphogen sonic hedgehog collaborates with netrin-1 to guide axons in the spinal cord.Trends Neurosci. 2003 Dec;26(12):641-3. doi: 10.1016/j.tins.2003.09.006. Trends Neurosci. 2003. PMID: 14624844 Review.
Cited by
-
Netrin-1 promotes the vasculogenic capacity of human adipose-derived stem cells.Cell Tissue Bank. 2023 Jun;24(2):357-367. doi: 10.1007/s10561-022-10038-0. Epub 2022 Oct 12. Cell Tissue Bank. 2023. PMID: 36222969
-
Hypoxia-Elicited Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Alleviate Myocardial Infarction by Promoting Angiogenesis through the miR-214/Sufu Pathway.Stem Cells Int. 2023 Jan 13;2023:1662182. doi: 10.1155/2023/1662182. eCollection 2023. Stem Cells Int. 2023. PMID: 39280589 Free PMC article.
-
Pleiotropic beneficial effects of sonic hedgehog gene therapy in an experimental model of peripheral limb ischemia.Mol Ther. 2011 Apr;19(4):658-66. doi: 10.1038/mt.2010.292. Epub 2011 Jan 11. Mol Ther. 2011. PMID: 21224834 Free PMC article.
-
Sonic Hedgehog upregulation does not enhance the survival and engraftment of stem cell-derived cardiomyocytes in infarcted hearts.PLoS One. 2020 Jan 16;15(1):e0227780. doi: 10.1371/journal.pone.0227780. eCollection 2020. PLoS One. 2020. PMID: 31945113 Free PMC article.
-
Controlled delivery of sonic hedgehog morphogen and its potential for cardiac repair.PLoS One. 2013 May 14;8(5):e63075. doi: 10.1371/journal.pone.0063075. Print 2013. PLoS One. 2013. PMID: 23690982 Free PMC article.
References
-
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–38. - PubMed
-
- Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–85. - PubMed
-
- Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620–4. - PubMed
-
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

