Inhibition of transcription by platinum antitumor compounds
- PMID: 20046924
- PMCID: PMC2752884
- DOI: 10.1039/b907567d
Inhibition of transcription by platinum antitumor compounds
Abstract
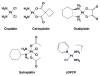
Cisplatin, carboplatin, and oxaliplatin are three FDA-approved members of the platinum anticancer drug family. These compounds induce apoptosis in tumor cells by binding to nuclear DNA, forming a variety of structural adducts and triggering cellular responses, one of which is the inhibition of transcription. In this report we present (i) a detailed review of the structural investigations of various Pt-DNA adducts and the effects of these lesions on global DNA geometry; (ii) research detailing inhibition of cellular transcription by Pt-DNA adducts; and (iii) a mechanistic analysis of how DNA structural distortions induced by platinum damage may inhibit RNA synthesis in vivo. A thorough understanding of the molecular mechanism of action of platinum antitumor agents will aid in the development of new compounds in the family.
Figures

Similar articles
-
Transcription inhibition by platinum-DNA cross-links in live mammalian cells.J Am Chem Soc. 2010 Jun 2;132(21):7429-35. doi: 10.1021/ja101495v. J Am Chem Soc. 2010. PMID: 20443565 Free PMC article.
-
Monofunctional platinum-DNA adducts are strong inhibitors of transcription and substrates for nucleotide excision repair in live mammalian cells.Cancer Res. 2012 Feb 1;72(3):790-800. doi: 10.1158/0008-5472.CAN-11-3151. Epub 2011 Dec 16. Cancer Res. 2012. PMID: 22180496 Free PMC article.
-
Multiple states of stalled T7 RNA polymerase at DNA lesions generated by platinum anticancer agents.J Biol Chem. 2003 Dec 26;278(52):52084-92. doi: 10.1074/jbc.M310120200. Epub 2003 Oct 8. J Biol Chem. 2003. PMID: 14534300
-
Cellular processing of platinum anticancer drugs.Nat Rev Drug Discov. 2005 Apr;4(4):307-20. doi: 10.1038/nrd1691. Nat Rev Drug Discov. 2005. PMID: 15789122 Review.
-
Cellular and molecular pharmacology of oxaliplatin.Mol Cancer Ther. 2002 Jan;1(3):227-35. Mol Cancer Ther. 2002. PMID: 12467217 Review.
Cited by
-
DEWNA: dynamic entropy weight network analysis and its application to the DNA-binding proteome in A549 cells with cisplatin-induced damage.Brief Bioinform. 2024 Sep 23;25(6):bbae564. doi: 10.1093/bib/bbae564. Brief Bioinform. 2024. PMID: 39487085 Free PMC article.
-
Mitochondrial DNA damage and its consequences for mitochondrial gene expression.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):979-91. doi: 10.1016/j.bbagrm.2012.06.002. Epub 2012 Jun 19. Biochim Biophys Acta. 2012. PMID: 22728831 Free PMC article. Review.
-
In vitro anticancer activity of cis-diammineplatinum(II) complexes with β-diketonate leaving group ligands.J Med Chem. 2012 Jun 14;55(11):5326-36. doi: 10.1021/jm3002857. Epub 2012 May 18. J Med Chem. 2012. PMID: 22606945 Free PMC article.
-
Anticancer Activity of Metallodrugs and Metallizing Host Defense Peptides-Current Developments in Structure-Activity Relationship.Int J Mol Sci. 2024 Jul 3;25(13):7314. doi: 10.3390/ijms25137314. Int J Mol Sci. 2024. PMID: 39000421 Free PMC article. Review.
-
Synthesis of [PtCl2(4,4'-dialkoxy-2,2'-bipyridine)] complexes and their in vitro anticancer properties.Metallomics. 2013 Aug;5(8):973-87. doi: 10.1039/c3mt00128h. Metallomics. 2013. PMID: 23817622 Free PMC article.
References
-
- Loehrer PJ, Einhorn LH. Ann Intern Med. 1984;100:704–713. - PubMed
-
- Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, III, Walker JL, Gersell D. New Eng J Med. 1999;340:1154–1161. - PubMed
-
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. New Eng J Med. 1999;340:1137–1143. - PubMed
-
- Bosl GJ, Bajorin DF, Sheinfeld J, Motzer RJ, Chaganti RSK. In: Cancer: Principles & practice of oncology. 6. DeVita VT Jr, Hellman S, Rosenberg SA, editors. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1491–1518.
-
- Bosl GJ, Motzer RJ. New Eng J Med. 1997;337:242–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

