Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy
- PMID: 20041693
- PMCID: PMC2815556
- DOI: 10.1021/bi901723p
Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy
Abstract
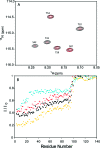
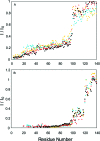
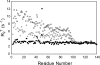
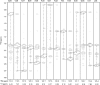
Three familial variants of the presynaptic protein alpha-synuclein (alphaS), A30P, E46K, and A53T, correlate with rare inherited Parkinson's disease (PD), while wild-type alphaS is implicated in sporadic PD. The classic manifestation of both familiar and sporadic PD is the formation of fibrillar structures of alphaS which accumulate as the main component in intraneuronal Lewy bodies. At presynaptic termini, the partitioning of alphaS between disordered cytosolic and membrane-bound states likely mediates its proposed role in regulation of reserve pools of synaptic vesicles. Previously, we reported on multiple distinct phospholipid binding modes of alphaS with slow binding kinetics. Here, we report the phospholipid binding properties of the disease variants, viewed by solution NMR in a residue-specific manner. Our results agree qualitatively with previous biophysical studies citing overall decreased lipid affinity for the A30P mutation, comparable affinity for A53T, and an increased level of binding of E46K, relative to wild-type alphaS. Additionally, our NMR results describe the distribution of lipid-bound states for alphaS: the population of the SL1 binding mode (residues 3-25 bound as a helix) is augmented by each of the disease variants, relative to wild-type alphaS. We propose that the SL1 binding mode, which anchors the N-terminus of alphaS in the lipoprotein complex while the hydrophobic NAC region remains dynamically disordered, is prone to intermolecular interactions which progress toward disease-associated oligomers and fibrils. The elevation of the SL1 binding mode, unchecked by a proportionate population of binding modes incorporating the full N-terminal domain, may well account for the increased toxicity of the A30P, E46K, and A53T disease variants of alphaS.
Figures

Similar articles
-
E46K Parkinson's-linked mutation enhances C-terminal-to-N-terminal contacts in alpha-synuclein.J Mol Biol. 2009 May 22;388(5):1022-32. doi: 10.1016/j.jmb.2009.03.065. Epub 2009 Apr 5. J Mol Biol. 2009. PMID: 19345692 Free PMC article.
-
Structures and free energy landscapes of the A53T mutant-type α-synuclein protein and impact of A53T mutation on the structures of the wild-type α-synuclein protein with dynamics.ACS Chem Neurosci. 2013 Jul 17;4(7):1101-13. doi: 10.1021/cn400041j. Epub 2013 May 17. ACS Chem Neurosci. 2013. PMID: 23607785 Free PMC article.
-
Familial Parkinson disease mutations influence α-synuclein assembly.Neurobiol Dis. 2011 Sep;43(3):715-24. doi: 10.1016/j.nbd.2011.05.025. Epub 2011 Jun 7. Neurobiol Dis. 2011. PMID: 21684335
-
Disruptive membrane interactions of alpha-synuclein aggregates.Biochim Biophys Acta Proteins Proteom. 2019 May;1867(5):468-482. doi: 10.1016/j.bbapap.2018.10.006. Epub 2018 Oct 11. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 30315896 Review.
-
Interactions of α-synuclein oligomers with lipid membranes.Biochim Biophys Acta Biomembr. 2021 Apr 1;1863(4):183536. doi: 10.1016/j.bbamem.2020.183536. Epub 2020 Dec 26. Biochim Biophys Acta Biomembr. 2021. PMID: 33373595 Review.
Cited by
-
Role of Parkinson's Disease-Linked Mutations and N-Terminal Acetylation on the Oligomerization of α-Synuclein Induced by 3,4-Dihydroxyphenylacetaldehyde.ACS Chem Neurosci. 2019 Jan 16;10(1):690-703. doi: 10.1021/acschemneuro.8b00498. Epub 2018 Nov 5. ACS Chem Neurosci. 2019. PMID: 30352158 Free PMC article.
-
Restricting α-synuclein transport into mitochondria by inhibition of α-synuclein-VDAC complexation as a potential therapeutic target for Parkinson's disease treatment.Cell Mol Life Sci. 2022 Jun 19;79(7):368. doi: 10.1007/s00018-022-04389-w. Cell Mol Life Sci. 2022. PMID: 35718804 Free PMC article.
-
Regulation of exocytosis and mitochondrial relocalization by Alpha-synuclein in a mammalian cell model.NPJ Parkinsons Dis. 2019 Jun 27;5:12. doi: 10.1038/s41531-019-0084-6. eCollection 2019. NPJ Parkinsons Dis. 2019. PMID: 31263746 Free PMC article.
-
The Molecular Basis of the Sodium Dodecyl Sulfate Effect on Human Ubiquitin Structure: A Molecular Dynamics Simulation Study.Sci Rep. 2018 Feb 1;8(1):2150. doi: 10.1038/s41598-018-20669-7. Sci Rep. 2018. PMID: 29391595 Free PMC article.
-
Exploring the role of post-translational modifications in regulating α-synuclein interactions by studying the effects of phosphorylation on nanobody binding.Protein Sci. 2018 Jul;27(7):1262-1274. doi: 10.1002/pro.3412. Protein Sci. 2018. PMID: 29603451 Free PMC article.
References
-
- Perrin R. J.; Woods W. S.; Clayton D. F.; George J. M. (2001) Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 276, 41958–41962. - PubMed
-
- Zhu M.; Fink A. L. (2003) Lipid binding inhibits α-synuclein fibril formation. J. Biol. Chem. 278, 16873–16877. - PubMed
-
- Zhu M.; Li J.; Fink A. L. (2003) The association of α-synuclein with membranes affects bilayer structure, stability, and fibril formation. J. Biol. Chem. 278, 40186–40197. - PubMed
-
- Necula M.; Chirita C. N.; Kuret J. (2003) Rapid anionic micelle-mediated α-synuclein fibrillization in vitro. J. Biol. Chem. 278, 46674–46680. - PubMed
-
- Welch K.; Yuan J. Y. (2003) α-Synuclein oligomerization: A role for lipids?. Trends Neurosci. 26, 517–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

