The gut microbiota modulates host energy and lipid metabolism in mice
- PMID: 20040631
- PMCID: PMC2853437
- DOI: 10.1194/jlr.M002774
The gut microbiota modulates host energy and lipid metabolism in mice
Abstract
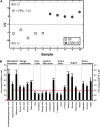
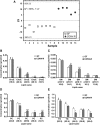
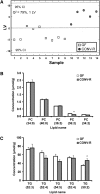
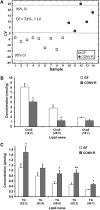
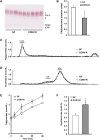
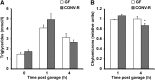
The gut microbiota has recently been identified as an environmental factor that may promote metabolic diseases. To investigate the effect of gut microbiota on host energy and lipid metabolism, we compared the serum metabolome and the lipidomes of serum, adipose tissue, and liver of conventionally raised (CONV-R) and germ-free mice. The serum metabolome of CONV-R mice was characterized by increased levels of energy metabolites, e.g., pyruvic acid, citric acid, fumaric acid, and malic acid, while levels of cholesterol and fatty acids were reduced. We also showed that the microbiota modified a number of lipid species in the serum, adipose tissue, and liver, with its greatest effect on triglyceride and phosphatidylcholine species. Triglyceride levels were lower in serum but higher in adipose tissue and liver of CONV-R mice, consistent with increased lipid clearance. Our findings show that the gut microbiota affects both host energy and lipid metabolism and highlights its role in the development of metabolic diseases.
Figures

Similar articles
-
Metabolomics characterization of energy metabolism reveals glycogen accumulation in gut-microbiota-lacking mice.J Nutr Biochem. 2012 Jul;23(7):752-8. doi: 10.1016/j.jnutbio.2011.03.019. Epub 2011 Aug 12. J Nutr Biochem. 2012. PMID: 21840188
-
Gut microbiota modulate the metabolism of brown adipose tissue in mice.J Proteome Res. 2012 Feb 3;11(2):620-30. doi: 10.1021/pr200938v. Epub 2011 Nov 21. J Proteome Res. 2012. PMID: 22053906
-
Gut microbiota affects lens and retinal lipid composition.Exp Eye Res. 2009 Nov;89(5):604-7. doi: 10.1016/j.exer.2009.06.018. Epub 2009 Jul 8. Exp Eye Res. 2009. PMID: 19591827
-
Effects of the gut microbiota on obesity and glucose homeostasis.Trends Endocrinol Metab. 2011 Apr;22(4):117-23. doi: 10.1016/j.tem.2011.01.002. Epub 2011 Feb 23. Trends Endocrinol Metab. 2011. PMID: 21353592 Review.
-
Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism.J Intern Med. 2010 Oct;268(4):320-8. doi: 10.1111/j.1365-2796.2010.02270.x. J Intern Med. 2010. PMID: 21050286 Review.
Cited by
-
Effects of Interactions between Feeding Patterns and the Gut Microbiota on Pig Reproductive Performance.Animals (Basel). 2024 Sep 19;14(18):2714. doi: 10.3390/ani14182714. Animals (Basel). 2024. PMID: 39335303 Free PMC article.
-
What defines a healthy gut microbiome?Gut. 2024 Oct 7;73(11):1893-1908. doi: 10.1136/gutjnl-2024-333378. Gut. 2024. PMID: 39322314 Free PMC article. Review.
-
Mechanisms of hepatic and renal injury in lipid metabolism disorders in metabolic syndrome.Int J Biol Sci. 2024 Sep 9;20(12):4783-4798. doi: 10.7150/ijbs.100394. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309427 Free PMC article. Review.
-
Dual Regulation Mechanism of Obesity: DNA Methylation and Intestinal Flora.Biomedicines. 2024 Jul 23;12(8):1633. doi: 10.3390/biomedicines12081633. Biomedicines. 2024. PMID: 39200098 Free PMC article. Review.
-
Gut Microbiota and Metabolic Syndrome: Relationships and Opportunities for New Therapeutic Strategies.Scientifica (Cairo). 2024 Jul 15;2024:4222083. doi: 10.1155/2024/4222083. eCollection 2024. Scientifica (Cairo). 2024. PMID: 39041052 Free PMC article. Review.
References
-
- Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. 2006. Microbial ecology: human gut microbes associated with obesity. Nature. 444: 1022–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

