Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3
- PMID: 20038637
- PMCID: PMC3991695
- DOI: 10.4049/jimmunol.0903218
Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3
Abstract
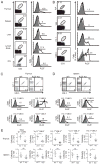
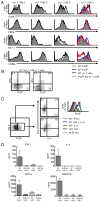
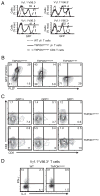
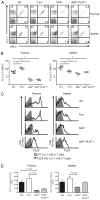
The broad-complex tramtrack and bric a brac-zinc finger transcriptional regulator (BTB-ZF), promyelocytic leukemia zinc finger (PLZF), was recently shown to control the development of the characteristic innate T cell phenotype and effector functions of NK T cells. Interestingly, the ectopic expression of PLZF was shown to push conventional T cells into an activated state that seems to be proinflammatory. The factors that control the normal expression of PLZF in lymphocytes are unknown. In this study, we show that PLZF expression is not restricted to NK T cells but is also expressed by a subset of gammadelta T cells, functionally defining distinct subsets of this innate T cell population. A second BTB-ZF gene, ThPOK, is important for the phenotype of the PLZF-expressing gammadelta T cells. Most importantly, TCR signal strength and expression of inhibitor of differentiation gene 3 control the frequency of PLZF-expressing gammadelta T cells. This study defines the factors that control the propensity of the immune system to produce potentially disease-causing T cell subsets.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

Similar articles
-
TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity.Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12453-8. doi: 10.1073/pnas.0903895106. Epub 2009 Jul 15. Proc Natl Acad Sci U S A. 2009. PMID: 19617548 Free PMC article.
-
PLZF Controls the Development of Fetal-Derived IL-17+Vγ6+ γδ T Cells.J Immunol. 2015 Nov 1;195(9):4273-81. doi: 10.4049/jimmunol.1500939. Epub 2015 Sep 25. J Immunol. 2015. PMID: 26408661 Free PMC article.
-
Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling.J Immunol. 2011 May 15;186(10):5801-6. doi: 10.4049/jimmunol.1100119. Epub 2011 Apr 8. J Immunol. 2011. PMID: 21478405 Free PMC article.
-
Development of PLZF-expressing innate T cells.Curr Opin Immunol. 2011 Apr;23(2):220-7. doi: 10.1016/j.coi.2010.12.016. Epub 2011 Jan 21. Curr Opin Immunol. 2011. PMID: 21257299 Free PMC article. Review.
-
Promyelocytic leukemia zinc finger controls type 2 immune responses in the lungs by regulating lineage commitment and the function of innate and adaptive immune cells.Int Immunopharmacol. 2024 Mar 30;130:111670. doi: 10.1016/j.intimp.2024.111670. Epub 2024 Feb 18. Int Immunopharmacol. 2024. PMID: 38373386 Review.
Cited by
-
Classical MHC expression by DP thymocytes impairs the selection of non-classical MHC restricted innate-like T cells.Nat Commun. 2021 Apr 16;12(1):2308. doi: 10.1038/s41467-021-22589-z. Nat Commun. 2021. PMID: 33863906 Free PMC article.
-
Intrinsic factors and CD1d1 but not CD1d2 expression levels control invariant natural killer T cell subset differentiation.Nat Commun. 2023 Dec 1;14(1):7922. doi: 10.1038/s41467-023-43424-7. Nat Commun. 2023. PMID: 38040679 Free PMC article.
-
SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics.Immunity. 2010 Aug 27;33(2):203-15. doi: 10.1016/j.immuni.2010.07.013. Epub 2010 Jul 30. Immunity. 2010. PMID: 20674402 Free PMC article.
-
TCR-mediated ThPOK induction promotes development of mature (CD24-) gammadelta thymocytes.EMBO J. 2010 Jul 21;29(14):2329-41. doi: 10.1038/emboj.2010.113. Epub 2010 Jun 15. EMBO J. 2010. PMID: 20551904 Free PMC article.
-
Developmental origins of murine γδ T-cell subsets.Immunology. 2019 Apr;156(4):299-304. doi: 10.1111/imm.13032. Epub 2019 Jan 21. Immunology. 2019. PMID: 30552818 Free PMC article. Review.
References
-
- Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging β- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. - PubMed
-
- Hayday AC. [γ][δ] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–386. - PubMed
-
- Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

