The biological effect of an antisense oligonucleotide depends on its route of endocytosis and trafficking
- PMID: 20038250
- PMCID: PMC2883474
- DOI: 10.1089/oli.2009.0211
The biological effect of an antisense oligonucleotide depends on its route of endocytosis and trafficking
Abstract
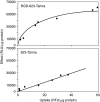
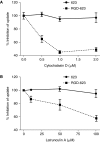
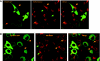
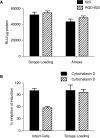
We demonstrate that the biological effect of an oligonucleotide is influenced by its route of cellular uptake. Utilizing a splice-switching antisense oligonucleotide (SSO) and a sensitive reporter assay involving correction of RNA splicing, we examined induction of luciferase in cells treated either with various concentrations of an unconjugated ("free") SSO or an SSO conjugated to a bivalent RGD ligand that promotes binding to the alphavbeta3 integrin (RGD-SSO). Under conditions of equal accumulation in cells, the RGD-SSO consistently had a greater effect on luciferase induction than the unconjugated SSO. We determined that the RGD-SSO and the unconjugated SSO were internalized by distinct endocytotic pathways, suggesting that the route of internalization affects the magnitude of the biological response.
Figures

Similar articles
-
Targeted delivery of a splice-switching oligonucleotide by cationic polyplexes of RGD-oligonucleotide conjugate.Mol Pharm. 2012 May 7;9(5):1502-10. doi: 10.1021/mp300113c. Epub 2012 Apr 25. Mol Pharm. 2012. PMID: 22497548 Free PMC article.
-
Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands.J Am Chem Soc. 2010 Jul 7;132(26):8848-9. doi: 10.1021/ja102635c. J Am Chem Soc. 2010. PMID: 20550198 Free PMC article.
-
Intracellular delivery of an anionic antisense oligonucleotide via receptor-mediated endocytosis.Nucleic Acids Res. 2008 May;36(8):2764-76. doi: 10.1093/nar/gkn115. Epub 2008 Mar 26. Nucleic Acids Res. 2008. PMID: 18367474 Free PMC article.
-
Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides.J Drug Target. 2013 Jan;21(1):27-43. doi: 10.3109/1061186X.2012.740674. Epub 2012 Nov 19. J Drug Target. 2013. PMID: 23163768 Free PMC article. Review.
-
Splice-switching antisense oligonucleotides as therapeutic drugs.Nucleic Acids Res. 2016 Aug 19;44(14):6549-63. doi: 10.1093/nar/gkw533. Epub 2016 Jun 10. Nucleic Acids Res. 2016. PMID: 27288447 Free PMC article. Review.
Cited by
-
The chemistry and biology of oligonucleotide conjugates.Acc Chem Res. 2012 Jul 17;45(7):1067-76. doi: 10.1021/ar2002123. Epub 2012 Feb 21. Acc Chem Res. 2012. PMID: 22353142 Free PMC article.
-
Chemical Manipulation of the Endosome Trafficking Machinery: Implications for Oligonucleotide Delivery.Biomedicines. 2021 May 5;9(5):512. doi: 10.3390/biomedicines9050512. Biomedicines. 2021. PMID: 34063104 Free PMC article. Review.
-
Peptide-mediated Cell and In Vivo Delivery of Antisense Oligonucleotides and siRNA.Mol Ther Nucleic Acids. 2012 Jun 12;1(6):e27. doi: 10.1038/mtna.2012.18. Mol Ther Nucleic Acids. 2012. PMID: 23344079 Free PMC article. No abstract available.
-
Covalent conjugation of oligonucleotides with cell-targeting ligands.Bioorg Med Chem. 2013 Oct 15;21(20):6217-23. doi: 10.1016/j.bmc.2013.05.037. Epub 2013 Jun 1. Bioorg Med Chem. 2013. PMID: 23777829 Free PMC article.
-
Aptamer-mediated delivery of splice-switching oligonucleotides to the nuclei of cancer cells.Nucleic Acid Ther. 2012 Jun;22(3):187-95. doi: 10.1089/nat.2012.0347. Nucleic Acid Ther. 2012. PMID: 22703281 Free PMC article.
References
-
- ABES S. MOULTON H. TURNER J. CLAIR P. RICHARD J.P. IVERSEN P. GAIT M.J. LEBLEU B. Peptide-based delivery of nucleic acids: design, mechanism of uptake and applications to splice-correcting oligonucleotides. Biochem. Soc. Trans. 2007;35:53–55. - PubMed
-
- APLIN A.E. JULIANO R.L. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell. Sci. 1999;112(Pt 5):695–706. - PubMed
-
- BENDIFALLAH N. RASMUSSEN F.W. ZACHAR V. EBBESEN P. NIELSEN P.E. KOPPELHUS U. Evaluation of cell-penetrating peptides (CPPs) as vehicles for intracellular delivery of antisense peptide nucleic acid (PNA) Bioconjug. Chem. 2006;17:750–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

