Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection
- PMID: 20037649
- PMCID: PMC2793027
- DOI: 10.1371/journal.pone.0008439
Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection
Abstract
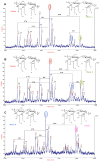
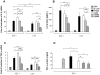
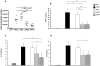
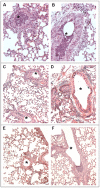
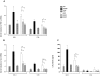
Pseudomonas aeruginosa can establish life-long airways chronic infection in patients with cystic fibrosis (CF) with pathogenic variants distinguished from initially acquired strain. Here, we analysed chemical and biological activity of P. aeruginosa Pathogen-Associated Molecular Patterns (PAMPs) in clonal strains, including mucoid and non-mucoid phenotypes, isolated during a period of up to 7.5 years from a CF patient. Chemical structure by MS spectrometry defined lipopolysaccharide (LPS) lipid A and peptidoglycan (PGN) muropeptides with specific structural modifications temporally associated with CF lung infection. Gene sequence analysis revealed novel mutation in pagL, which supported lipid A changes. Both LPS and PGN had different potencies when activating host innate immunity via binding TLR4 and Nod1. Significantly higher NF-kB activation, IL-8 expression and production were detected in HEK293hTLR4/MD2-CD14 and HEK293hNod1 after stimulation with LPS and PGN respectively, purified from early P. aeruginosa strain as compared to late strains. Similar results were obtained in macrophages-like cells THP-1, epithelial cells of CF origin IB3-1 and their isogenic cells C38, corrected by insertion of cystic fibrosis transmembrane conductance regulator (CFTR). In murine model, altered LPS structure of P. aeruginosa late strains induces lower leukocyte recruitment in bronchoalveolar lavage and MIP-2, KC and IL-1beta cytokine levels in lung homogenates when compared with early strain. Histopathological analysis of lung tissue sections confirmed differences between LPS from early and late P. aeruginosa. Finally, in this study for the first time we unveil how P. aeruginosa has evolved the capacity to evade immune system detection, thus promoting survival and establishing favourable conditions for chronic persistence. Our findings provide relevant information with respect to chronic infections in CF.
Conflict of interest statement
Figures
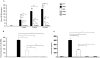
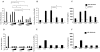
Similar articles
-
Pseudomonas aeruginosa Lipid A Structural Variants Induce Altered Immune Responses.Am J Respir Cell Mol Biol. 2024 Aug;71(2):207-218. doi: 10.1165/rcmb.2024-0059OC. Am J Respir Cell Mol Biol. 2024. PMID: 38656811
-
Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Jun 30;7:243. doi: 10.3389/fcimb.2017.00243. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713772 Free PMC article. Review.
-
Mucoid Pseudomonas aeruginosa and regional inflammation in the cystic fibrosis lung.J Cyst Fibros. 2019 Nov;18(6):796-803. doi: 10.1016/j.jcf.2019.04.009. Epub 2019 Apr 26. J Cyst Fibros. 2019. PMID: 31036488 Free PMC article.
-
Mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis.Pediatr Pulmonol. 2000 Nov;30(5):413-24. doi: 10.1002/1099-0496(200011)30:5<413::aid-ppul8>3.0.co;2-9. Pediatr Pulmonol. 2000. PMID: 11064433 Review.
-
IL-17 primes airway epithelial cells lacking functional Cystic Fibrosis Transmembrane conductance Regulator (CFTR) to increase NOD1 responses.Biochem Biophys Res Commun. 2010 Jan 1;391(1):505-9. doi: 10.1016/j.bbrc.2009.11.088. Epub 2009 Nov 20. Biochem Biophys Res Commun. 2010. PMID: 19931506
Cited by
-
Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung.PLoS One. 2013 May 16;8(5):e63237. doi: 10.1371/journal.pone.0063237. Print 2013. PLoS One. 2013. PMID: 23696800 Free PMC article.
-
Host-guest chemistry of the peptidoglycan.J Med Chem. 2010 Jul 8;53(13):4813-29. doi: 10.1021/jm100086u. J Med Chem. 2010. PMID: 20524613 Free PMC article. No abstract available.
-
Dewetting-induced formation and mechanical properties of synthetic bacterial outer membrane models (GUVs) with controlled inner-leaflet lipid composition.Soft Matter. 2019 May 15;15(19):3938-3948. doi: 10.1039/c9sm00223e. Soft Matter. 2019. PMID: 31011738 Free PMC article.
-
Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo.PLoS One. 2011;6(10):e26525. doi: 10.1371/journal.pone.0026525. Epub 2011 Oct 18. PLoS One. 2011. PMID: 22028895 Free PMC article.
-
Adaptation of Pseudomonas aeruginosa in Cystic Fibrosis airways influences virulence of Staphylococcus aureus in vitro and murine models of co-infection.PLoS One. 2014 Mar 6;9(3):e89614. doi: 10.1371/journal.pone.0089614. eCollection 2014. PLoS One. 2014. PMID: 24603807 Free PMC article.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jéhanno M, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. - PubMed
-
- Girardin SE, Travassos LH, Hervé M, Blanot D, Boneca IG, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. - PubMed
-
- Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

