Emerging roles of ATF2 and the dynamic AP1 network in cancer
- PMID: 20029425
- PMCID: PMC2874064
- DOI: 10.1038/nrc2681
Emerging roles of ATF2 and the dynamic AP1 network in cancer
Erratum in
- Nat Rev Cancer. 2010 May;10(5):379
Abstract
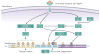
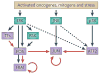
Cooperation among transcription factors is central for their ability to execute specific transcriptional programmes. The AP1 complex exemplifies a network of transcription factors that function in unison under normal circumstances and during the course of tumour development and progression. This Perspective summarizes our current understanding of the changes in members of the AP1 complex and the role of ATF2 as part of this complex in tumorigenesis.
Conflict of interest statement
Figures
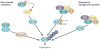
Similar articles
-
[Ap1-like cis-elements in 5'-regulatory region of human apolipoprotein A-I gene].Mol Biol (Mosk). 2008 Mar-Apr;42(2):295-305. Mol Biol (Mosk). 2008. PMID: 18610838 Russian.
-
Transient receptor potential melastatin-3 (TRPM3)-induced activation of AP-1 requires Ca2+ ions and the transcription factors c-Jun, ATF2, and ternary complex factor.Mol Pharmacol. 2015 Apr;87(4):617-28. doi: 10.1124/mol.114.095695. Epub 2015 Jan 9. Mol Pharmacol. 2015. PMID: 25576487
-
Mutual regulation of c-Jun and ATF2 by transcriptional activation and subcellular localization.EMBO J. 2006 Mar 8;25(5):1058-69. doi: 10.1038/sj.emboj.7601020. Epub 2006 Mar 2. EMBO J. 2006. PMID: 16511568 Free PMC article.
-
ATF2, a paradigm of the multifaceted regulation of transcription factors in biology and disease.Pharmacol Res. 2017 May;119:347-357. doi: 10.1016/j.phrs.2017.02.004. Epub 2017 Feb 15. Pharmacol Res. 2017. PMID: 28212892 Free PMC article. Review.
-
The roles of ATF2 (activating transcription factor 2) in tumorigenesis.Biochem Soc Trans. 2012 Feb;40(1):230-4. doi: 10.1042/BST20110630. Biochem Soc Trans. 2012. PMID: 22260696 Review.
Cited by
-
FOSL1 promotes metastasis of head and neck squamous cell carcinoma through super-enhancer-driven transcription program.Mol Ther. 2021 Aug 4;29(8):2583-2600. doi: 10.1016/j.ymthe.2021.03.024. Epub 2021 Mar 29. Mol Ther. 2021. PMID: 33794365 Free PMC article.
-
Emerging roles of activating transcription factor 2 in the development of breast cancer: a comprehensive review.Precis Clin Med. 2023 Oct 25;6(4):pbad028. doi: 10.1093/pcmedi/pbad028. eCollection 2023 Dec. Precis Clin Med. 2023. PMID: 37955015 Free PMC article. Review.
-
Rough set soft computing cancer classification and network: one stone, two birds.Cancer Inform. 2010 Jul 15;9:139-45. doi: 10.4137/cin.s4874. Cancer Inform. 2010. PMID: 20706619 Free PMC article.
-
The potential of activator protein 1 (AP-1) in cancer targeted therapy.Front Immunol. 2023 Jul 6;14:1224892. doi: 10.3389/fimmu.2023.1224892. eCollection 2023. Front Immunol. 2023. PMID: 37483616 Free PMC article. Review.
-
Syk: a new target for attenuation of Helicobacter pylori-induced gastric mucosal inflammatory responses.Inflammopharmacology. 2019 Apr;27(2):203-211. doi: 10.1007/s10787-019-00577-6. Epub 2019 Feb 28. Inflammopharmacology. 2019. PMID: 30820719 Review.
References
-
- Lee W, Mitchell P, Tjian R. Purified transcription factor AP1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. - PubMed
-
- Angel P, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. - PubMed
-
- Wisdom R. AP1: One switch for many signals. Exp Cell Res. 1999;253:180–185. - PubMed
-
- Eferl R, Wagner EF. AP1: a double-edged sword in tumorigenesis. Nature Rev Cancer. 2003;3:859–868. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos and the AP1 complex in cell-proliferation and transformation. Biochem Biophys Acta. 1991;1072:129–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA051995/CA/NCI NIH HHS/United States
- R01 CA059908-12/CA/NCI NIH HHS/United States
- R01 CA051995/CA/NCI NIH HHS/United States
- CA117927/CA/NCI NIH HHS/United States
- R01 CA099961/CA/NCI NIH HHS/United States
- R01 CA099961-06A2/CA/NCI NIH HHS/United States
- R01 CA117927/CA/NCI NIH HHS/United States
- R01 CA059908/CA/NCI NIH HHS/United States
- CA099961/CA/NCI NIH HHS/United States
- T32 CA121929/CA/NCI NIH HHS/United States
- R01 CA117927-04/CA/NCI NIH HHS/United States
- R01 CA051995-18/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

