Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation
- PMID: 20029400
- PMCID: PMC2871075
- DOI: 10.1038/pcan.2009.55
Novel role for the transient receptor potential channel TRPM2 in prostate cancer cell proliferation
Abstract
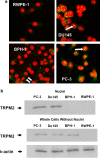
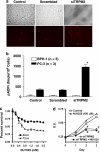
We have identified a novel function for a member of the transient receptor potential (TRP) protein super-family, TRPM2, in prostate cancer cell proliferation. TRPM2 encodes a non-selective cation-permeable ion channel. We found that selectively knocking down TRPM2 with the small interfering RNA technique inhibited the growth of prostate cancer cells but not of non-cancerous cells. The subcellular localization of this protein is also remarkably different between cancerous and non-cancerous cells. In BPH-1 (benign), TRPM2 protein is homogenously located near the plasma membrane and in the cytoplasm, whereas in the cancerous cells (PC-3 and DU-145), a significant amount of the TRPM2 protein is located in the nuclei in a clustered pattern. Furthermore, we have found that TRPM2 inhibited nuclear ADP-ribosylation in prostate cancer cells. However, TRPM2 knockdown-induced inhibition of proliferation is independent of the activity of poly(ADP-ribose) polymerases. We conclude that TRPM2 is essential for prostate cancer cell proliferation and may be a potential target for the selective treatment of prostate cancer.
Figures

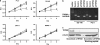
Similar articles
-
Methods for Investigating Transient Receptor Potential Melastatin-2 (TRPM2): A Cation Channel Activated by ADP-Ribose and Involved in Cell Death.Methods Mol Biol. 2023;2609:213-226. doi: 10.1007/978-1-0716-2891-1_13. Methods Mol Biol. 2023. PMID: 36515838
-
The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma.Sci Rep. 2016 Dec 23;6:38471. doi: 10.1038/srep38471. Sci Rep. 2016. PMID: 28008929 Free PMC article.
-
TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase.Br J Pharmacol. 2004 Sep;143(1):186-92. doi: 10.1038/sj.bjp.0705914. Epub 2004 Aug 9. Br J Pharmacol. 2004. PMID: 15302683 Free PMC article.
-
TRPM2 and TRPM7: channel/enzyme fusions to generate novel intracellular sensors.Pflugers Arch. 2005 Oct;451(1):220-7. doi: 10.1007/s00424-005-1444-0. Epub 2005 Jul 7. Pflugers Arch. 2005. PMID: 16001276 Review.
-
TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose.Pflugers Arch. 2005 Oct;451(1):212-9. doi: 10.1007/s00424-005-1446-y. Epub 2005 Jun 11. Pflugers Arch. 2005. PMID: 15952035 Review.
Cited by
-
Non-coding RNAs as therapeutic targets in cancer and its clinical application.J Pharm Anal. 2024 Jul;14(7):100947. doi: 10.1016/j.jpha.2024.02.001. Epub 2024 Feb 8. J Pharm Anal. 2024. PMID: 39149142 Free PMC article. Review.
-
Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks?Philos Trans R Soc Lond B Biol Sci. 2014 Feb 3;369(1638):20130097. doi: 10.1098/rstb.2013.0097. Print 2014 Mar 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24493745 Free PMC article. Review.
-
Roles of NAD+ and Its Metabolites Regulated Calcium Channels in Cancer.Molecules. 2020 Oct 20;25(20):4826. doi: 10.3390/molecules25204826. Molecules. 2020. PMID: 33092205 Free PMC article. Review.
-
Different regulation of PARP1, PARP2, PARP3 and TRPM2 genes expression in acute myeloid leukemia cells.BMC Cancer. 2020 May 18;20(1):435. doi: 10.1186/s12885-020-06903-4. BMC Cancer. 2020. PMID: 32423430 Free PMC article.
-
Scalaradial Is a Potent Inhibitor of Transient Receptor Potential Melastatin 2 (TRPM2) Ion Channels.J Nat Prod. 2017 Oct 27;80(10):2741-2750. doi: 10.1021/acs.jnatprod.7b00515. Epub 2017 Oct 11. J Nat Prod. 2017. PMID: 29019677 Free PMC article.
References
-
- Pervarskaya N, Flourakis M, Bidaux G, Thebault S, Skryma R. Differential role of TRP channels in prostate cancer. Biochem Soc Trans. 2007;35:133–135. - PubMed
-
- Ishii M, Oyama A, Hagiwara T, Miyazaki A, Mori Y, Kiuchi Y, et al. Facilitation of H2O2-induced A172 human glioblastoma cell death by insertion of oxidative stress-sensitive TRPM2 channels. Anticancer Res. 2007;27:3987–3992. - PubMed
-
- Orfanelli U, Wenke A-K, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res. 2008;18:1128–1140. - PubMed
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Perraud AL, Schmitz C, Scharenberg AM. TRPM2 Ca2+ permeable cation channels: from gene to biological function. Cell Calcium. 2003;33:519–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

