Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia
- PMID: 20028456
- PMCID: PMC2819586
- DOI: 10.1111/j.1471-4159.2009.06552.x
Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia
Abstract
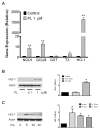
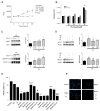
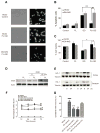
Many phytochemicals function as noxious agents that protect plants against insects and other damaging organisms. However, at subtoxic doses, the same phytochemicals may activate adaptive cellular stress response pathways that can protect cells against a variety of adverse conditions. We screened a panel of botanical pesticides using cultured human and rodent neuronal cell models, and identified plumbagin as a novel potent activator of the nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway. In vitro, plumbagin increases nuclear localization and transcriptional activity of Nrf2, and induces the expression of the Nrf2/ARE-dependent genes, such as heme oxygenase 1 in human neuroblastoma cells. Plumbagin specifically activates the Nrf2/ARE pathway in primary mixed cultures from ARE-human placental alkaline phosphatase reporter mice. Exposure of neuroblastoma cells and primary cortical neurons to plumbagin provides protection against subsequent oxidative and metabolic insults. The neuroprotective effects of plumbagin are abolished by RNA interference-mediated knockdown of Nrf2 expression. In vivo, administration of plumbagin significantly reduces the amount of brain damage and ameliorates-associated neurological deficits in a mouse model of focal ischemic stroke. Our findings establish precedence for the identification and characterization of neuroprotective phytochemicals based upon their ability to activate adaptive cellular stress response pathways.
Figures


Similar articles
-
S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo.J Neurochem. 2015 Apr;133(2):298-308. doi: 10.1111/jnc.12986. Epub 2015 Jan 23. J Neurochem. 2015. PMID: 25393425
-
Neuroprotection by acetyl-11-keto-β-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway.Sci Rep. 2014 Nov 11;4:7002. doi: 10.1038/srep07002. Sci Rep. 2014. PMID: 25384416 Free PMC article.
-
α-Lipoic Acid Promotes Neurological Recovery After Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage.Cell Physiol Biochem. 2017;43(3):1273-1287. doi: 10.1159/000481840. Epub 2017 Oct 9. Cell Physiol Biochem. 2017. PMID: 28992629
-
Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin.Mol Cells. 2009 Mar 31;27(3):279-82. doi: 10.1007/s10059-009-0050-y. Epub 2009 Mar 19. Mol Cells. 2009. PMID: 19326073 Free PMC article. Review.
-
Neuroprotective Potential of Brown Seaweed Phytochemicals in Rodent Models of Cerebral Ischemia.J Med Food. 2023 Jul;26(7):436-444. doi: 10.1089/jmf.2023.0058. Epub 2023 Jul 3. J Med Food. 2023. PMID: 37405739 Review.
Cited by
-
Plumbagin inhibits tumorigenesis and angiogenesis of ovarian cancer cells in vivo.Int J Cancer. 2013 Mar 1;132(5):1201-12. doi: 10.1002/ijc.27724. Epub 2012 Jul 27. Int J Cancer. 2013. PMID: 22806981 Free PMC article.
-
Brain metabolism in health, aging, and neurodegeneration.EMBO J. 2017 Jun 1;36(11):1474-1492. doi: 10.15252/embj.201695810. Epub 2017 Apr 24. EMBO J. 2017. PMID: 28438892 Free PMC article. Review.
-
Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies.Antioxidants (Basel). 2023 Apr 13;12(4):920. doi: 10.3390/antiox12040920. Antioxidants (Basel). 2023. PMID: 37107295 Free PMC article. Review.
-
Mitochondrial function in human neuroblastoma cells is up-regulated and protected by NQO1, a plasma membrane redox enzyme.PLoS One. 2013 Jul 11;8(7):e69030. doi: 10.1371/journal.pone.0069030. Print 2013. PLoS One. 2013. PMID: 23874855 Free PMC article.
-
Pharmacological Features and Therapeutic Implications of Plumbagin in Cancer and Metabolic Disorders: A Narrative Review.Nutrients. 2024 Sep 8;16(17):3033. doi: 10.3390/nu16173033. Nutrients. 2024. PMID: 39275349 Free PMC article. Review.
References
-
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a cap ‘n’ Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. - PubMed
-
- Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. - PubMed
-
- Calixto JB, Kassuya CA, Andre E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208. - PubMed
-
- Camandola S, Cutler RG, Gary DS, Milhavet O, Mattson MP. Suppression of calcium release from IP3-sensitive stores mediates the anti-apoptostic function of NF-κB. J Biol Chem. 2005;280:22287–22296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

