Pathogenic cysteine mutations affect progranulin function and production of mature granulins
- PMID: 20028451
- PMCID: PMC2819556
- DOI: 10.1111/j.1471-4159.2009.06546.x
Pathogenic cysteine mutations affect progranulin function and production of mature granulins
Abstract
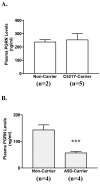
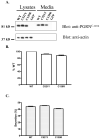
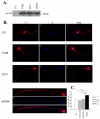
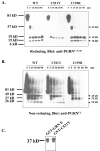
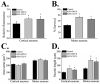
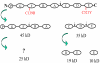
Frontotemporal dementia with ubiquitin-positive inclusions (FTLD-U) can be caused by mutations in the progranulin gene (GRN). Progranulin (PGRN) is a cysteine-rich growth factor, which is proteolytically cleaved by elastase to produce several granulins (GRNs). All FTLD-U mutations in GRN characterized to date result in reduced secreted PGRN protein. We recently reported a Spanish family with progressive non-fluent aphasia and dementia in which a novel C521Y mutation segregates with disease. A second cysteine mutation (C139R) has also been reported to be disease specific. Allele-specific mRNA expression assays in brain reveal that the C521Y mutant allele is expressed at similar levels to the wild-type allele. Furthermore, plasma PGRN levels in C521Y carriers are comparable with non-carrier family relatives, suggesting that the mutation does not affect PGRN protein expression and secretion in vivo. Despite normal PGRN levels C521Y and C139R mutant GRNs show reduced neurite growth-stimulating activity in vitro. Further study revealed that these mutations also cause impaired cleavage of PGRN by elastase. Our data suggest that these mutations affect the function of full-length PGRN as well as elastase cleavage of PGRN into GRNs, leading to neurodegeneration.
Figures

Similar articles
-
Intracellular Proteolysis of Progranulin Generates Stable, Lysosomal Granulins that Are Haploinsufficient in Patients with Frontotemporal Dementia Caused by GRN Mutations.eNeuro. 2017 Aug 18;4(4):ENEURO.0100-17.2017. doi: 10.1523/ENEURO.0100-17.2017. eCollection 2017 Jul-Aug. eNeuro. 2017. PMID: 28828399 Free PMC article.
-
Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations.Acta Neuropathol Commun. 2020 Oct 7;8(1):163. doi: 10.1186/s40478-020-01037-x. Acta Neuropathol Commun. 2020. PMID: 33028409 Free PMC article.
-
Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: a novel therapeutic lead to treat frontotemporal dementia.Mol Neurodegener. 2016 Jun 24;11(1):46. doi: 10.1186/s13024-016-0114-3. Mol Neurodegener. 2016. PMID: 27341800 Free PMC article.
-
Progranulin in frontotemporal lobar degeneration and neuroinflammation.J Neuroinflammation. 2007 Feb 11;4:7. doi: 10.1186/1742-2094-4-7. J Neuroinflammation. 2007. PMID: 17291356 Free PMC article. Review.
-
Progranulin as a therapeutic target for dementia.Expert Opin Ther Targets. 2018 Jul;22(7):579-585. doi: 10.1080/14728222.2018.1487951. Epub 2018 Jun 22. Expert Opin Ther Targets. 2018. PMID: 29889573 Review.
Cited by
-
Progranulin: an emerging target for FTLD therapies.Brain Res. 2012 Jun 26;1462:118-28. doi: 10.1016/j.brainres.2012.01.047. Epub 2012 Jan 28. Brain Res. 2012. PMID: 22338605 Free PMC article. Review.
-
Lysosomal Dysfunction and Other Pathomechanisms in FTLD: Evidence from Progranulin Genetics and Biology.Adv Exp Med Biol. 2021;1281:219-242. doi: 10.1007/978-3-030-51140-1_14. Adv Exp Med Biol. 2021. PMID: 33433878 Free PMC article.
-
The African Turquoise Killifish Genome Provides Insights into Evolution and Genetic Architecture of Lifespan.Cell. 2015 Dec 3;163(6):1539-54. doi: 10.1016/j.cell.2015.11.008. Cell. 2015. PMID: 26638078 Free PMC article.
-
Genetics screening in an Italian cohort of patients with Amyotrophic Lateral Sclerosis: the importance of early testing and its implication.J Neurol. 2024 Apr;271(4):1921-1936. doi: 10.1007/s00415-023-12142-x. Epub 2023 Dec 19. J Neurol. 2024. PMID: 38112783
-
Peripheral GRN mRNA and Serum Progranulin Levels as a Potential Indicator for Both the Presence of Splice Site Mutations and Individuals at Risk for Frontotemporal Dementia.J Alzheimers Dis. 2019;67(1):159-167. doi: 10.3233/JAD-180599. J Alzheimers Dis. 2019. PMID: 30475763 Free PMC article.
References
-
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Bernardi L, Tomaino C, Anfossi M, et al. Novel PSEN1 and PGRN mutations in early-onset familial frontotemporal dementia. Neurobiol. Aging. 2009;30:1825–1833. - PubMed
-
- Brouwers N, Sleegers K, Engelborghs S, et al. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology. 2008;71:656–664. - PubMed
-
- Cruchaga C, Fernandez-Seara MA, Seijo-Martinez M, et al. Cortical Atrophy and Language Network Reorganization Associated with a Novel Progranulin Mutation. Cereb. Cortex. 2009;19:1751–1760. - PubMed
-
- Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

