VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification
- PMID: 20026664
- PMCID: PMC2812533
- DOI: 10.1084/jem.20091837
VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification
Abstract
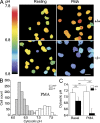
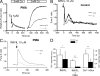
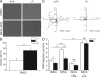
Neutrophils kill microbes with reactive oxygen species generated by the NADPH oxidase, an enzyme which moves electrons across membranes. Voltage-gated proton channels (voltage-sensing domain only protein [VSOP]/Hv1) are required for high-level superoxide production by phagocytes, but the mechanism of this effect is not established. We show that neutrophils from VSOP/Hv1-/- mice lack proton currents but have normal electron currents, indicating that these cells have a fully functional oxidase that cannot conduct protons. VSOP/Hv1-/- neutrophils had a more acidic cytosol, were more depolarized, and produced less superoxide and hydrogen peroxide than neutrophils from wild-type mice. Hydrogen peroxide production was rescued by providing an artificial conductance with gramicidin. Loss of VSOP/Hv1 also aborted calcium responses to chemoattractants, increased neutrophil spreading, and decreased neutrophil migration. The migration defect was restored by the addition of a calcium ionophore. Our findings indicate that proton channels extrude the acid and compensate the charge generated by the oxidase, thereby sustaining calcium entry signals that control the adhesion and motility of neutrophils. Loss of proton channels thus aborts superoxide production and causes a severe signaling defect in neutrophils.
Figures
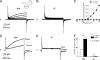

Similar articles
-
Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes?Mol Cell Endocrinol. 2012 Apr 28;353(1-2):82-7. doi: 10.1016/j.mce.2011.10.005. Epub 2011 Oct 26. Mol Cell Endocrinol. 2012. PMID: 22056415 Review.
-
Regulation of Neutrophil Functions by Hv1/VSOP Voltage-Gated Proton Channels.Int J Mol Sci. 2021 Mar 5;22(5):2620. doi: 10.3390/ijms22052620. Int J Mol Sci. 2021. PMID: 33807711 Free PMC article. Review.
-
Hv1/VSOP regulates neutrophil directional migration and ERK activity by tuning ROS production.J Leukoc Biol. 2020 May;107(5):819-831. doi: 10.1002/JLB.2A0320-110RR. Epub 2020 Apr 17. J Leukoc Biol. 2020. PMID: 32303121
-
Intracellular shunting of O2(-) contributes to charge compensation and preservation of neutrophil respiratory burst in the absence of voltage-gated proton channel activity.Exp Cell Res. 2013 Jul 15;319(12):1875-1888. doi: 10.1016/j.yexcr.2013.03.031. Epub 2013 Apr 8. Exp Cell Res. 2013. PMID: 23578765 Free PMC article.
-
Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst.Proc Natl Acad Sci U S A. 2009 May 5;106(18):7642-7. doi: 10.1073/pnas.0902761106. Epub 2009 Apr 16. Proc Natl Acad Sci U S A. 2009. PMID: 19372380 Free PMC article.
Cited by
-
Nicotine inhibits activation of microglial proton currents via interactions with α7 acetylcholine receptors.J Physiol Sci. 2017 Jan;67(1):235-245. doi: 10.1007/s12576-016-0460-5. Epub 2016 Jun 2. J Physiol Sci. 2017. PMID: 27256075 Free PMC article.
-
Insights into the structure and function of HV1 from a meta-analysis of mutation studies.J Gen Physiol. 2016 Aug;148(2):97-118. doi: 10.1085/jgp.201611619. J Gen Physiol. 2016. PMID: 27481712 Free PMC article. Review.
-
Protection from acute lung injury by a peptide designed to inhibit the voltage-gated proton channel.iScience. 2022 Dec 29;26(1):105901. doi: 10.1016/j.isci.2022.105901. eCollection 2023 Jan 20. iScience. 2022. PMID: 36660473 Free PMC article.
-
Redox regulation of store-operated Ca2+ entry.Antioxid Redox Signal. 2014 Aug 20;21(6):915-32. doi: 10.1089/ars.2013.5615. Epub 2013 Dec 18. Antioxid Redox Signal. 2014. PMID: 24053140 Free PMC article. Review.
-
Control of intracellular pH and bicarbonate by CO2 diffusion into human sperm.Nat Commun. 2023 Sep 5;14(1):5395. doi: 10.1038/s41467-023-40855-0. Nat Commun. 2023. PMID: 37669933 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

