Chaperone-mediated autophagy in health and disease
- PMID: 20026330
- PMCID: PMC2843772
- DOI: 10.1016/j.febslet.2009.12.025
Chaperone-mediated autophagy in health and disease
Abstract
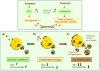
Chaperone-mediated autophagy (CMA) is a lysosomal pathway that participates in the degradation of cytosolic proteins. CMA is activated by starvation and in response to stressors that result in protein damage. The selectivity intrinsic to CMA allows for removal of damaged proteins without disturbing nearby functional ones. CMA works in a coordinated manner with other autophagic pathways, which can compensate for each other. Interest in CMA has recently grown because of the connections established between this autophagic pathway and human pathologies. Here we review the unique properties of CMA compared to other autophagic pathways and its relevance in health and disease.
Copyright 2009 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Chaperone-mediated autophagy in aging and disease.Curr Top Dev Biol. 2006;73:205-35. doi: 10.1016/S0070-2153(05)73007-6. Curr Top Dev Biol. 2006. PMID: 16782460 Review.
-
Chaperone-mediated autophagy: selectivity pays off.Trends Endocrinol Metab. 2010 Mar;21(3):142-50. doi: 10.1016/j.tem.2009.10.003. Epub 2009 Oct 24. Trends Endocrinol Metab. 2010. PMID: 19857975 Free PMC article. Review.
-
Chaperone mediated autophagy in aging: Starve to prosper.Ageing Res Rev. 2016 Dec;32:13-21. doi: 10.1016/j.arr.2016.07.001. Epub 2016 Jul 30. Ageing Res Rev. 2016. PMID: 27484893 Review.
-
Chaperone-mediated autophagy.Proc Am Thorac Soc. 2010 Feb;7(1):29-39. doi: 10.1513/pats.200909-102JS. Proc Am Thorac Soc. 2010. PMID: 20160146 Free PMC article. Review.
-
Chaperone-mediated autophagy: molecular mechanisms and physiological relevance.Semin Cell Dev Biol. 2010 Sep;21(7):719-26. doi: 10.1016/j.semcdb.2010.02.005. Epub 2010 Feb 20. Semin Cell Dev Biol. 2010. PMID: 20176123 Free PMC article. Review.
Cited by
-
The role of autophagy in Parkinson's disease.Neural Regen Res. 2012 Jan 15;7(2):141-5. doi: 10.3969/j.issn.1673-5374.2012.02.011. Neural Regen Res. 2012. PMID: 25767490 Free PMC article. Review.
-
Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: implications for the lysosomal glycocalyx.BMC Biol. 2012 Jul 19;10:62. doi: 10.1186/1741-7007-10-62. BMC Biol. 2012. PMID: 22809326 Free PMC article.
-
A selective type of autophagy to maintain glioma stem cell activity.Stem Cell Investig. 2023 Jan 6;10:1. doi: 10.21037/sci-2022-047. eCollection 2023. Stem Cell Investig. 2023. PMID: 36742282 Free PMC article. No abstract available.
-
Autophagy paradox and ceramide.Biochim Biophys Acta. 2014 May;1841(5):783-92. doi: 10.1016/j.bbalip.2013.09.005. Epub 2013 Sep 19. Biochim Biophys Acta. 2014. PMID: 24055889 Free PMC article. Review.
-
Nutrition and healthy ageing: calorie restriction or polyphenol-rich "MediterrAsian" diet?Oxid Med Cell Longev. 2013;2013:707421. doi: 10.1155/2013/707421. Epub 2013 Aug 28. Oxid Med Cell Longev. 2013. PMID: 24069505 Free PMC article. Review.
References
-
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. - PubMed
-
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;18:895–899. - PubMed
-
- Dice J. Chaperone-mediated autophagy. Autophagy. 2007;3:295–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 NS038370-060002/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- NS038370/NS/NINDS NIH HHS/United States
- P01 DK041918/DK/NIDDK NIH HHS/United States
- AG031782/AG/NIA NIH HHS/United States
- R37 AG021904/AG/NIA NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R01 AG021904/AG/NIA NIH HHS/United States
- DK041918/DK/NIDDK NIH HHS/United States
- P01 AG031782-01A1/AG/NIA NIH HHS/United States
- R01 AG021904-07/AG/NIA NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- AG021904/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

