Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-Linked beta-N-acetylglucosamine in 3T3-L1 adipocytes
- PMID: 20018868
- PMCID: PMC2820748
- DOI: 10.1074/jbc.M109.077818
Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-Linked beta-N-acetylglucosamine in 3T3-L1 adipocytes
Abstract
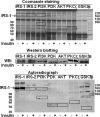
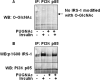
Increased O-linked beta-N-acetylglucosamine (O-GlcNAc) is associated with insulin resistance in muscle and adipocytes. Upon insulin treatment of insulin-responsive adipocytes, O-GlcNAcylation of several proteins is increased. Key insulin signaling proteins, including IRS-1, IRS-2, and PDK1, are substrates for OGT, suggesting potential O-GlcNAc control points within the pathway. To elucidate the roles of O-GlcNAc in dampening insulin signaling (Vosseller, K., Wells, L., Lane, M. D., and Hart, G. W. (2002) Proc. Natl. Acad. Sci. U. S. A. 99, 5313-5318), we focused on the pathway upstream of AKT. Increasing O-GlcNAc in 3T3-L1 adipocytes decreases phosphoinositide 3-kinase (PI3K) interactions with both IRS-1 and IRS-2. Elevated O-GlcNAc also reduces phosphorylation of the PI3K p85 binding motifs (YXXM) of IRS-1 and results in a concomitant reduction in tyrosine phosphorylation of Y(608)XXM in IRS-1, one of the two main PI3K p85 binding motifs. Additionally, insulin signaling stimulates the interaction of OGT with PDK1. We conclude that one of the steps at which O-GlcNAc contributes to insulin resistance is by inhibiting phosphorylation at the Y(608)XXM PI3K p85 binding motif in IRS-1 and possibly at PDK1 as well.
Figures


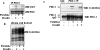

Similar articles
-
Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes.Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5313-8. doi: 10.1073/pnas.072072399. Proc Natl Acad Sci U S A. 2002. PMID: 11959983 Free PMC article.
-
O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes.Exp Mol Med. 2005 Jun 30;37(3):220-9. doi: 10.1038/emm.2005.30. Exp Mol Med. 2005. PMID: 16000877
-
O-linked N-acetylglucosamine modification of insulin receptor substrate-1 occurs in close proximity to multiple SH2 domain binding motifs.Mol Cell Proteomics. 2009 Dec;8(12):2733-45. doi: 10.1074/mcp.M900207-MCP200. Epub 2009 Aug 11. Mol Cell Proteomics. 2009. PMID: 19671924 Free PMC article.
-
Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes.Endocrinology. 2005 Mar;146(3):1328-37. doi: 10.1210/en.2004-0777. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576463
-
Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes.Diabetes. 2001 Aug;50(8):1891-900. doi: 10.2337/diabetes.50.8.1891. Diabetes. 2001. PMID: 11473053
Cited by
-
The Beginner's Guide to O-GlcNAc: From Nutrient Sensitive Pathway Regulation to Its Impact on the Immune System.Front Immunol. 2022 Jan 31;13:828648. doi: 10.3389/fimmu.2022.828648. eCollection 2022. Front Immunol. 2022. PMID: 35173739 Free PMC article. Review.
-
Chitin research revisited.Mar Drugs. 2010 Jun 28;8(7):1988-2012. doi: 10.3390/md8071988. Mar Drugs. 2010. PMID: 20714419 Free PMC article. Review.
-
Discovery and confirmation of O-GlcNAcylated proteins in rat liver mitochondria by combination of mass spectrometry and immunological methods.PLoS One. 2013 Oct 2;8(10):e76399. doi: 10.1371/journal.pone.0076399. eCollection 2013. PLoS One. 2013. PMID: 24098488 Free PMC article.
-
O-GlcNAc Transferase Is Essential for Sensory Neuron Survival and Maintenance.J Neurosci. 2017 Feb 22;37(8):2125-2136. doi: 10.1523/JNEUROSCI.3384-16.2017. Epub 2017 Jan 23. J Neurosci. 2017. PMID: 28115479 Free PMC article.
-
Diabetes Pharmacotherapy and its effects on the Skeletal Muscle Energy Metabolism.Mini Rev Med Chem. 2024;24(16):1470-1480. doi: 10.2174/0113895575299439240216081711. Mini Rev Med Chem. 2024. PMID: 38549524 Review.
References
-
- Whelan S. A., Hart G. W. (2003) Circ. Res. 93, 1047–1058 - PubMed
-
- Wells L., Vosseller K., Hart G. W. (2001) Science 291, 2376–2378 - PubMed
-
- Zachara N. E., Hart G. W. (2006) Biochim. Biophys. Acta 1761, 599–617 - PubMed
-
- Comer F. I., Hart G. W. (2001) Biochemistry 40, 7845–7852 - PubMed
-
- Lefebvre T., Alonso C., Mahboub S., Dupire M. J., Zanetta J. P., Caillet-Boudin M. L., Michalski J. C. (1999) Biochim. Biophys. Acta 1472, 71–81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

