Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding
- PMID: 20018855
- PMCID: PMC2836088
- DOI: 10.1074/jbc.M109.081828
Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding
Abstract
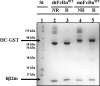
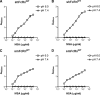
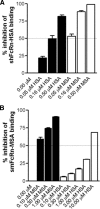
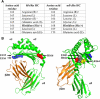
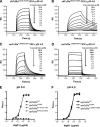
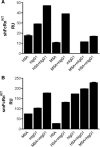
The neonatal Fc receptor (FcRn) regulates the serum half-life of both IgG and albumin through a pH-dependent mechanism that involves salvage from intracellular degradation. Therapeutics and diagnostics built on IgG, Fc, and albumin fusions are frequently evaluated in rodents regarding biodistribution and pharmacokinetics. Thus, it is important to address cross-species ligand reactivity with FcRn, because in vivo testing of such molecules is done in the presence of competing murine ligands, both in wild type (WT) and human FcRn (hFcRn) transgenic mice. Here, binding studies were performed in vitro using enzyme-linked immunosorbent assay and surface plasmon resonance with recombinant soluble forms of human (shFcRn(WT)) and mouse (smFcRn(WT)) receptors. No binding of albumin from either species was observed at physiological pH to either receptor. At acidic pH, a 100-fold difference in binding affinity was observed. Specifically, smFcRn(WT) bound human serum albumin with a K(D) of approximately 90 microM, whereas shFcRn(WT) bound mouse serum albumin with a K(D) of 0.8 microM. shFcRn(WT) ignored mouse IgG1, and smFcRn(WT) bound strongly to human IgG1. The latter pair also interacted at physiological pH with calculated affinity in the micromolar range. In all cases, binding of albumin and IgG from either species to both receptors were additive. Cross-species albumin binding differences could partly be explained by non-conserved amino acids found within the alpha2-domain of the receptor. Such distinct cross-species FcRn binding differences must be taken into consideration when IgG- and albumin-based therapeutics and diagnostics are evaluated in rodents for their pharmacokinetics.
Figures



Similar articles
-
A strategy for bacterial production of a soluble functional human neonatal Fc receptor.J Immunol Methods. 2008 Feb 29;331(1-2):39-49. doi: 10.1016/j.jim.2007.11.003. Epub 2007 Dec 10. J Immunol Methods. 2008. PMID: 18155020
-
Albumin binding to FcRn: distinct from the FcRn-IgG interaction.Biochemistry. 2006 Apr 18;45(15):4983-90. doi: 10.1021/bi052628y. Biochemistry. 2006. PMID: 16605266
-
Extended plasma half-life of albumin-binding domain fused human IgA upon pH-dependent albumin engagement of human FcRn in vitro and in vivo.MAbs. 2021 Jan-Dec;13(1):1893888. doi: 10.1080/19420862.2021.1893888. MAbs. 2021. PMID: 33691596 Free PMC article.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
The immunologic functions of the neonatal Fc receptor for IgG.J Clin Immunol. 2013 Jan;33 Suppl 1(Suppl 1):S9-17. doi: 10.1007/s10875-012-9768-y. Epub 2012 Sep 5. J Clin Immunol. 2013. PMID: 22948741 Free PMC article. Review.
Cited by
-
Selection of IgG Variants with Increased FcRn Binding Using Random and Directed Mutagenesis: Impact on Effector Functions.Front Immunol. 2015 Feb 4;6:39. doi: 10.3389/fimmu.2015.00039. eCollection 2015. Front Immunol. 2015. PMID: 25699055 Free PMC article.
-
Mechanistic incorporation of FcRn binding in plasma and endosomes in a whole body PBPK model for large molecules.J Pharmacokinet Pharmacodyn. 2023 Jun;50(3):229-241. doi: 10.1007/s10928-023-09849-9. Epub 2023 Mar 6. J Pharmacokinet Pharmacodyn. 2023. PMID: 36877385
-
Cross-species analysis of Fc engineered anti-Lewis-Y human IgG1 variants in human neonatal receptor transgenic mice reveal importance of S254 and Y436 in binding human neonatal Fc receptor.MAbs. 2016 May-Jun;8(4):775-86. doi: 10.1080/19420862.2016.1156285. Epub 2016 Mar 30. MAbs. 2016. PMID: 27030023 Free PMC article.
-
Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs.MAbs. 2015;7(2):344-51. doi: 10.1080/19420862.2015.1008345. MAbs. 2015. PMID: 25654695 Free PMC article.
-
Single protein encapsulated SN38 for tumor-targeting treatment.J Transl Med. 2023 Dec 10;21(1):897. doi: 10.1186/s12967-023-04778-0. J Transl Med. 2023. PMID: 38072965 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

