Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma
- PMID: 20016540
- PMCID: PMC3934361
- DOI: 10.1038/gt.2009.161
Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma
Abstract
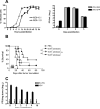
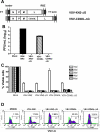
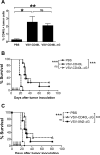
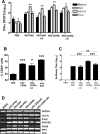
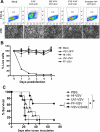
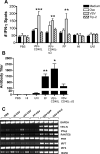
A fully intact immune system would be expected to hinder the efficacy of oncolytic virotherapy by inhibiting viral replication. Simultaneously, however, it may also enhance antitumor therapy through initiation of proinflammatory, antiviral cytokine responses at the tumor site. The aim of this study was to investigate the role of a fully intact immune system on the antitumor efficacy of an oncolytic virus. In this respect, injection of oncolytic vesicular stomatitis virus (VSV) into subcutaneous B16ova melanomas in C57Bl/6 mice leads to tumor regression, but it is not associated with viral replicative burst in the tumor. In contrast, intratumoral delivery of VSV induces an acute proinflammatory reaction, which quickly resolves concomitantly with virus clearance. Consistent with the hypothesis that therapy may not be dependent on the ability of VSV to undergo progressive rounds of replication, a single-cycle VSV is equally effective as a fully replication-competent VSV, whereas inactivated viruses do not generate therapy. Even though therapy is dependent on host CD8+ and natural killer cells, these effects are not associated with interferon-gamma-dependent responses against either the virus or tumor. There is, however, a strong correlation between viral gene expression, induction of proinflammatory reaction in the tumor and in vivo therapy. Overall, our results suggest that acute innate antiviral immune response, which rapidly clears VSV from B16ova tumors, is associated with the therapy observed in this model. Therefore, the antiviral immune response to an oncolytic virus mediates an intricate balance between safety, restriction of oncolysis and, potentially, significant immune-mediated antitumor therapy.
Figures



Similar articles
-
Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer.Cancer Res. 2010 Jun 1;70(11):4539-49. doi: 10.1158/0008-5472.CAN-09-4658. Epub 2010 May 18. Cancer Res. 2010. PMID: 20484025 Free PMC article.
-
Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus.Hum Gene Ther. 2010 Apr;21(4):439-50. doi: 10.1089/hum.2009.143. Hum Gene Ther. 2010. PMID: 19922169 Free PMC article.
-
VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling.Mol Ther. 2011 Jan;19(1):150-8. doi: 10.1038/mt.2010.225. Epub 2010 Oct 19. Mol Ther. 2011. PMID: 20959810 Free PMC article.
-
Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer.J Gen Virol. 2012 Dec;93(Pt 12):2529-2545. doi: 10.1099/vir.0.046672-0. Epub 2012 Oct 10. J Gen Virol. 2012. PMID: 23052398 Free PMC article. Review.
-
Immunovirotherapy Based on Recombinant Vesicular Stomatitis Virus: Where Are We?Front Immunol. 2022 Jun 28;13:898631. doi: 10.3389/fimmu.2022.898631. eCollection 2022. Front Immunol. 2022. PMID: 35837384 Free PMC article. Review.
Cited by
-
Zebrafish in dermatology: a comprehensive review of their role in investigating abnormal skin pigmentation mechanisms.Front Physiol. 2023 Nov 23;14:1296046. doi: 10.3389/fphys.2023.1296046. eCollection 2023. Front Physiol. 2023. PMID: 38074315 Free PMC article. Review.
-
Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer.Cancer Res. 2010 Jun 1;70(11):4539-49. doi: 10.1158/0008-5472.CAN-09-4658. Epub 2010 May 18. Cancer Res. 2010. PMID: 20484025 Free PMC article.
-
The Oncolytic Virus VSV-GP Is Effective against Malignant Melanoma.Viruses. 2018 Mar 2;10(3):108. doi: 10.3390/v10030108. Viruses. 2018. PMID: 29498639 Free PMC article.
-
The profile of tumor antigens which can be targeted by immunotherapy depends upon the tumor's anatomical site.Mol Ther. 2014 Nov;22(11):1936-48. doi: 10.1038/mt.2014.134. Epub 2014 Jul 25. Mol Ther. 2014. PMID: 25059678 Free PMC article.
-
Fusogenic vesicular stomatitis virus combined with natural killer T cell immunotherapy controls metastatic breast cancer.Breast Cancer Res. 2024 May 15;26(1):78. doi: 10.1186/s13058-024-01818-5. Breast Cancer Res. 2024. PMID: 38750591 Free PMC article.
References
-
- Balachandran S, Barber G. Vesicular stomatitis virus therapy of tumors. IUBMB Life. 2000;50:135–138. - PubMed
-
- Ebert O, Harbaran S, Shinozaki K, Woo SLC. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2004;12:350–358. - PubMed
-
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. - PubMed
-
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

