Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks
- PMID: 20015298
- PMCID: PMC2829200
- DOI: 10.1111/j.1476-5381.2009.00480.x
Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks
Abstract
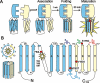
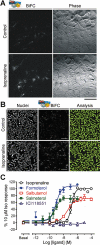
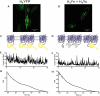
There is increasing complexity in the organization of seven transmembrane domain (7TM) receptor signalling pathways, and in the ability of their ligands to modulate and direct this signalling. Underlying these events is a network of protein interactions between the 7TM receptors themselves and associated effectors, such as G proteins and beta-arrestins. Bimolecular fluorescence complementation, or BiFC, is a technique capable of detecting these protein-protein events essential for 7TM receptor function. Fluorescent proteins, such as those from Aequorea victoria, are split into two non-fluorescent halves, which then tag the proteins under study. On association, these fragments refold and regenerate a mature fluorescent protein, producing a BiFC signal indicative of complex formation. Here, we review the experimental criteria for successful application of BiFC, considered in the context of 7TM receptor signalling events such as receptor dimerization, G protein and beta-arrestin signalling. The advantages and limitations of BiFC imaging are compared with alternative resonance energy transfer techniques. We show that the essential simplicity of the fluorescent BiFC measurement allows high-content and advanced imaging applications, and that it can probe more complex multi-protein interactions alone or in combination with resonance energy transfer. These capabilities suggest that BiFC techniques will become ever more useful in the analysis of ligand and 7TM receptor pharmacology at the molecular level of protein-protein interactions.
Figures

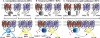
Similar articles
-
Dissecting the pharmacology of G protein-coupled receptor signaling complexes using bimolecular fluorescence complementation.Methods Mol Biol. 2012;897:109-38. doi: 10.1007/978-1-61779-909-9_6. Methods Mol Biol. 2012. PMID: 22674163
-
Quantitative analysis of neuropeptide Y receptor association with beta-arrestin2 measured by bimolecular fluorescence complementation.Br J Pharmacol. 2010 Jun;160(4):892-906. doi: 10.1111/j.1476-5381.2010.00676.x. Epub 2010 Apr 28. Br J Pharmacol. 2010. PMID: 20438572 Free PMC article.
-
Bimolecular fluorescence complementation analysis of inducible protein interactions: effects of factors affecting protein folding on fluorescent protein fragment association.J Mol Biol. 2009 Dec 4;394(3):391-409. doi: 10.1016/j.jmb.2009.08.069. Epub 2009 Sep 3. J Mol Biol. 2009. PMID: 19733184 Free PMC article.
-
Detecting and imaging protein-protein interactions during G protein-mediated signal transduction in vivo and in situ by using fluorescence-based techniques.Cell Biochem Biophys. 2006;45(1):85-109. doi: 10.1385/CBB:45:1:85. Cell Biochem Biophys. 2006. PMID: 16679566 Review.
-
Oligomerization of G protein-coupled receptors: biochemical and biophysical methods.Curr Med Chem. 2011;18(30):4606-34. doi: 10.2174/092986711797379285. Curr Med Chem. 2011. PMID: 21864280 Review.
Cited by
-
Efficient G protein coupling is not required for agonist-mediated internalization and membrane reorganization of the adenosine A3 receptor.FASEB J. 2021 Apr;35(4):e21211. doi: 10.1096/fj.202001729RR. FASEB J. 2021. PMID: 33710641 Free PMC article.
-
The use of fluorescence correlation spectroscopy to characterize the molecular mobility of fluorescently labelled G protein-coupled receptors.Biochem Soc Trans. 2016 Apr 15;44(2):624-9. doi: 10.1042/BST20150285. Biochem Soc Trans. 2016. PMID: 27068980 Free PMC article. Review.
-
HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation.Cells. 2020 Jan 22;9(2):272. doi: 10.3390/cells9020272. Cells. 2020. PMID: 31979118 Free PMC article.
-
A localized adaptor protein performs distinct functions at the Caulobacter cell poles.Proc Natl Acad Sci U S A. 2021 Mar 30;118(13):e2024705118. doi: 10.1073/pnas.2024705118. Proc Natl Acad Sci U S A. 2021. PMID: 33753507 Free PMC article.
-
Imaging--the interface with pharmacology: looking to the future.Br J Pharmacol. 2011 Aug;163(8):1563-4. doi: 10.1111/j.1476-5381.2011.01294.x. Br J Pharmacol. 2011. PMID: 21790531 Free PMC article.
References
-
- Anderie I, Schmid A. In vivo visualization of actin dynamics and actin interactions by BiFC. Cell Biol Int. 2007;31:1131–1135. - PubMed
-
- Berglund MM, Schober DA, Statnick MA, McDonald PH, Gehlert DR. The use of bioluminescence resonance energy transfer 2 to study neuropeptide Y receptor agonist-induced β-arrestin 2 interaction. J Pharmacol Exp Ther. 2003;306:147–156. - PubMed
-
- Briddon SJ, Gandia J, Amaral OB, Ferre S, Lluis C, Franco R, et al. Plasma membrane diffusion of G protein-coupled receptor oligomers. Biochim Biophys Acta. 2008;1783:2262–2268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

