HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy
- PMID: 20003286
- PMCID: PMC2803495
- DOI: 10.1186/1471-2407-9-433
HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy
Abstract
Background: The response rate of ovarian mucinous carcinomas to paclitaxel/carboplatin is low, prompting interest in targeted molecular therapies. We investigated HER2 expression and amplification, and the potential for trastuzumab therapy in this histologic subtype of ovarian cancer.
Methods: HER2 status was tested in 33 mucinous carcinomas and 16 mucinous borderline ovarian tumors (BOT)). Five cases with documented recurrence and with tissue from the recurrence available for testing were analyzed to determine whether HER2 amplification status changed over time. Three prospectively identified recurrent mucinous ovarian carcinomas were assessed for HER2 amplification and patients received trastuzumab therapy with conventional chemotherapy.
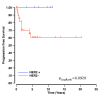
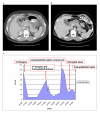
Results: Amplification of HER2 was observed in 6/33 (18.2%) mucinous carcinomas and 3/16 (18.8%) BOT. HER2 amplification in primary mucinous carcinomas was not associated with an increased likelihood of recurrence. The prospectively identified recurrent mucinous carcinomas showed overexpression and amplification of HER2; one patient's tumor responded dramatically to trastuzumab in combination with conventional chemotherapy, while another patient experienced an isolated central nervous system recurrence after trastuzumab therapy.
Conclusion: HER2 amplification is relatively common in ovarian mucinous carcinomas (6/33, 18.2%), although not of prognostic significance. Trastuzumab therapy is a treatment option for patients with mucinous carcinoma when the tumor has HER2 amplification and overexpression.
Figures

Similar articles
-
Molecular characterization of mucinous ovarian tumours supports a stratified treatment approach with HER2 targeting in 19% of carcinomas.J Pathol. 2013 Jan;229(1):111-20. doi: 10.1002/path.4088. J Pathol. 2013. PMID: 22899400
-
Targeting HER2 in ovarian and uterine cancers: challenges and future directions.Gynecol Oncol. 2014 Nov;135(2):364-70. doi: 10.1016/j.ygyno.2014.09.003. Epub 2014 Sep 16. Gynecol Oncol. 2014. PMID: 25220628 Review.
-
Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group.J Clin Oncol. 2003 Jan 15;21(2):283-90. doi: 10.1200/JCO.2003.10.104. J Clin Oncol. 2003. PMID: 12525520 Clinical Trial.
-
Pattern of HER-2 Gene Amplification and Protein Expression in Benign, Borderline, and Malignant Ovarian Serous and Mucinous Neoplasms.Int J Gynecol Pathol. 2017 Jan;36(1):50-57. doi: 10.1097/PGP.0000000000000302. Int J Gynecol Pathol. 2017. PMID: 27309616
-
Mucinous epithelial ovarian carcinoma.Ann Oncol. 2016 Apr;27 Suppl 1:i53-i57. doi: 10.1093/annonc/mdw087. Ann Oncol. 2016. PMID: 27141073 Review.
Cited by
-
Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden.J Pathol. 2015 Jun;236(2):201-9. doi: 10.1002/path.4516. Epub 2015 Mar 23. J Pathol. 2015. PMID: 25692284 Free PMC article.
-
Outcomes of non-high grade serous carcinoma after neoadjuvant chemotherapy for advanced-stage ovarian cancer: a Korean gynecologic oncology group study (OV 1708).BMC Cancer. 2019 Apr 11;19(1):341. doi: 10.1186/s12885-019-5514-7. BMC Cancer. 2019. PMID: 30971221 Free PMC article.
-
Epidemiology and survival outcomes of mucinous adenocarcinomas: A SEER population-based study.Sci Rep. 2018 Apr 17;8(1):6117. doi: 10.1038/s41598-018-24540-7. Sci Rep. 2018. PMID: 29666453 Free PMC article.
-
A Tri-part Protein Complementation System Using Antibody-Small Peptide Fusions Enables Homogeneous Immunoassays.Sci Rep. 2017 Aug 15;7(1):8186. doi: 10.1038/s41598-017-07569-y. Sci Rep. 2017. PMID: 28811487 Free PMC article.
-
Effects of Modulating Actin Dynamics on HER2 Cancer Cell Motility and Metastasis.Sci Rep. 2018 Nov 22;8(1):17243. doi: 10.1038/s41598-018-35284-9. Sci Rep. 2018. PMID: 30467396 Free PMC article.
References
-
- Winter W E III, Maxwell GL, Tian C, Carlson J W, Ozols R F, Rose P G, Markman M, Armstrong Deborah K, Muggia Franco, McGuire William P. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. - DOI - PubMed
-
- Pectasides D, Fonutrilas G, Aravantinos G, Kalofonos Haralampos P, Efstathiou E, Salamalekis E, Farmakis D, Skarlos D, Briasoulis E, Economopoulos T, Dimopoulos MA. Advanced stage mucinous epithelial ovarian cancer; the Hellenic Cooperative Oncology Group experience. Gynecol Oncol. 2005;99:7988–90. doi: 10.1016/j.ygyno.2005.07.022. - DOI - PubMed
-
- Tabrizi AD, Kalloger SE, Köbel M, Cipollone J, Roskelley CD, Mehl E, Gilks CB. Primary ovarian mucinous carcinoma of intestinal type. Analysis of histologic invasion patterns, survival and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Path. 2009. in press . - PubMed
-
- Pignata S, Ferrandina G, Scarfone G, Scollo P, Odicino F, Cormio G, Katsaros D, Villa A, Mereu L, Ghezzi F, Manzione L, Lauria R, Breda E, Alletti DG, Ballardini M, Lombardi A V, Sorio R, Mangili G, Priolo D, Magni G, Morabito A. Activity of chemotherapy in mucinous ovarian cancer with a recurrence free interval of more than 6 months: resuls from the SOCRATES restrospective study. BMC Cancer. 2008;8:252. doi: 10.1186/1471-2407-8-252. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

