MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein
- PMID: 19965586
- PMCID: PMC2853429
- DOI: 10.1194/jlr.M002345
MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein
Abstract
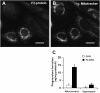
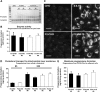
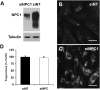
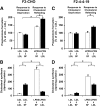
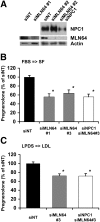
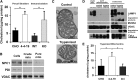
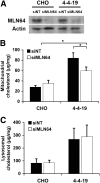
Niemann-Pick Type C (NPC) disease is a fatal, neurodegenerative disorder, caused in most cases by mutations in the late endosomal protein NPC1. A hallmark of NPC disease is endosomal cholesterol accumulation and an impaired cholesterol homeostatic response, which might affect cholesterol transport to mitochondria and, thus, mitochondrial and cellular function. This study aimed to characterize mitochondrial cholesterol homeostasis in NPC disease. Using wild-type and NPC1-deficient Chinese hamster ovary cells, stably transfected with a CYP11A1 complex to assess mitochondrial cholesterol import by pregnenolone production, we show that cholesterol transport to the mitochondrial inner membrane is not affected by loss of NPC1. However, mitochondrial cholesterol content was higher in NPC1-deficient than in wild-type cells. Cholesterol transport to the mitochondrial inner membrane increased markedly upon exposure of cholesterol-deprived cells to lipoproteins, indicating transport of endosomal cholesterol to mitochondria. Reduction of endosomal metastatic lymph node protein 64 (MLN64) by RNA interference decreased cholesterol transport to the mitochondrial inner membrane and reduced mitochondrial cholesterol levels in NPC1-deficient cells, suggesting that MLN64 transported cholesterol to mitochondria even in the absence of NPC1. In summary, this study describes a transport pathway for endosomal cholesterol to mitochondria that requires MLN64, but not NPC1, and that may be responsible for increased mitochondrial cholesterol in NPC disease.
Figures
Comment in
-
STARTing to understand MLN64 function in cholesterol transport.J Lipid Res. 2010 Aug;51(8):2015-7. doi: 10.1194/jlr.E008854. Epub 2010 May 28. J Lipid Res. 2010. PMID: 20511492 Free PMC article. No abstract available.
Similar articles
-
Adaptations of energy metabolism associated with increased levels of mitochondrial cholesterol in Niemann-Pick type C1-deficient cells.J Biol Chem. 2014 Jun 6;289(23):16278-89. doi: 10.1074/jbc.M114.559914. Epub 2014 Apr 30. J Biol Chem. 2014. PMID: 24790103 Free PMC article.
-
Niemann-Pick Type C2 protein contributes to the transport of endosomal cholesterol to mitochondria without interacting with NPC1.J Lipid Res. 2012 Dec;53(12):2632-42. doi: 10.1194/jlr.M029942. Epub 2012 Sep 7. J Lipid Res. 2012. PMID: 22962690 Free PMC article.
-
MLN64 induces mitochondrial dysfunction associated with increased mitochondrial cholesterol content.Redox Biol. 2017 Aug;12:274-284. doi: 10.1016/j.redox.2017.02.024. Epub 2017 Mar 2. Redox Biol. 2017. PMID: 28282615 Free PMC article.
-
The Niemann-Pick C proteins and trafficking of cholesterol through the late endosomal/lysosomal system.Curr Mol Med. 2002 Aug;2(5):485-505. doi: 10.2174/1566524023362375. Curr Mol Med. 2002. PMID: 12125814 Review.
-
Niemann-Pick type C mutations cause lipid traffic jam.Traffic. 2000 Mar;1(3):218-25. doi: 10.1034/j.1600-0854.2000.010304.x. Traffic. 2000. PMID: 11208105 Review.
Cited by
-
MLN64 transport to the late endosome is regulated by binding to 14-3-3 via a non-canonical binding site.PLoS One. 2012;7(4):e34424. doi: 10.1371/journal.pone.0034424. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514632 Free PMC article.
-
The role of endosomal cholesterol trafficking protein, StAR-related lipid transfer domain 3 (StarD3/MLN64), in BRIN-BD11 insulinoma cells.Protein Cell. 2016 Nov;7(11):833-838. doi: 10.1007/s13238-016-0315-0. Protein Cell. 2016. PMID: 27679500 Free PMC article. No abstract available.
-
Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System.Int J Mol Sci. 2020 Aug 13;21(16):5808. doi: 10.3390/ijms21165808. Int J Mol Sci. 2020. PMID: 32823559 Free PMC article. Review.
-
Genetic connections between neurological disorders and cholesterol metabolism.J Lipid Res. 2010 Sep;51(9):2489-503. doi: 10.1194/jlr.R006338. Epub 2010 May 13. J Lipid Res. 2010. PMID: 20466796 Free PMC article.
-
Cell cholesterol homeostasis: mediation by active cholesterol.Trends Cell Biol. 2010 Nov;20(11):680-7. doi: 10.1016/j.tcb.2010.08.007. Epub 2010 Sep 16. Trends Cell Biol. 2010. PMID: 20843692 Free PMC article. Review.
References
-
- Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277: 228–231. - PubMed
-
- Naureckiene S., Sleat D. E., Lackland H., Fensom A., Vanier M. T., Wattiaux R., Jadot M., Lobel P. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 290: 2298–2301. - PubMed
-
- Davies J. P., Ioannou Y. A. 2000. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem. 275: 24367–24374. - PubMed
-
- Millard E. E., Gale S. E., Dudley N., Zhang J., Schaffer J. E., Ory D. S. 2005. The sterol-sensing domain of the Niemann-Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J. Biol. Chem. 280: 28581–28590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

