The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila
- PMID: 19960024
- PMCID: PMC2822068
- DOI: 10.1038/cdd.2009.185
The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila
Abstract
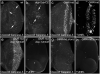
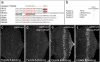
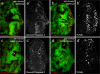
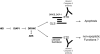
The cleaved-Caspase-3 antibody is a popular tool in apoptosis research in Drosophila. As the antibody was raised against cleaved human Caspase-3, it was assumed that it detects cleaved DRICE and DCP-1, Caspase-3-like effector caspases in Drosophila. However, as shown here, strong immunoreactivity persists in apoptotic models doubly mutant for drICE and dcp-1. In contrast, mutants of the apoptosome components DRONC (Caspase-9-like) and ARK (Apaf-1 related) do not label with the cleaved-Caspase-3 antibody. By peptide blocking experiments and further genetic studies, we provide evidence that the cleaved-Caspase-3 antibody recognizes multiple proteins including DCP-1 and likely DRICE, but also at least one additional unknown protein, all of which require DRONC for epitope exposure. The unknown substrate may be involved in non-apoptotic functions of DRONC. Because the cleaved-Caspase-3 antibody not only detects cleaved Caspase-3-like proteins in Drosophila, but also other proteins in a DRONC-dependent manner, it is more accurate to consider the cleaved-Caspase-3 antibody as a marker for DRONC activity, rather than effector caspase activity.
Figures
Similar articles
-
Activation mechanism and substrate specificity of the Drosophila initiator caspase DRONC.Cell Death Differ. 2008 May;15(5):938-45. doi: 10.1038/cdd.2008.23. Epub 2008 Feb 29. Cell Death Differ. 2008. PMID: 18309328
-
Cell survival and proliferation in Drosophila S2 cells following apoptotic stress in the absence of the APAF-1 homolog, ARK, or downstream caspases.Apoptosis. 2006 Apr;11(4):497-507. doi: 10.1007/s10495-006-5341-6. Apoptosis. 2006. PMID: 16532275
-
A biochemical analysis of the activation of the Drosophila caspase DRONC.Cell Death Differ. 2008 Mar;15(3):461-70. doi: 10.1038/sj.cdd.4402288. Epub 2007 Dec 14. Cell Death Differ. 2008. PMID: 18084239
-
Drosophila caspases as guardians of host-microbe interactions.Cell Death Differ. 2023 Feb;30(2):227-236. doi: 10.1038/s41418-022-01038-4. Epub 2022 Jul 9. Cell Death Differ. 2023. PMID: 35810247 Free PMC article. Review.
-
The fly caspases.Cell Death Differ. 2000 Nov;7(11):1039-44. doi: 10.1038/sj.cdd.4400756. Cell Death Differ. 2000. PMID: 11139276 Review.
Cited by
-
The secreted AdamTS-A metalloprotease is required for collective cell migration.Development. 2013 May;140(9):1981-93. doi: 10.1242/dev.087908. Epub 2013 Mar 27. Development. 2013. PMID: 23536567 Free PMC article.
-
Biological effects of the loss of homochirality in a multicellular organism.Nat Commun. 2022 Nov 18;13(1):7059. doi: 10.1038/s41467-022-34516-x. Nat Commun. 2022. PMID: 36400783 Free PMC article.
-
Characterization of an insecticidal toxin and pathogenicity of Pseudomonas taiwanensis against insects.PLoS Pathog. 2014 Aug 21;10(8):e1004288. doi: 10.1371/journal.ppat.1004288. eCollection 2014 Aug. PLoS Pathog. 2014. PMID: 25144637 Free PMC article.
-
Detecting Anastasis In Vivo by CaspaseTracker Biosensor.J Vis Exp. 2018 Feb 1;(132):54107. doi: 10.3791/54107. J Vis Exp. 2018. PMID: 29443051 Free PMC article.
-
The TORC1 inhibitors Nprl2 and Nprl3 mediate an adaptive response to amino-acid starvation in Drosophila.Cell Death Differ. 2014 Sep;21(9):1460-8. doi: 10.1038/cdd.2014.63. Epub 2014 May 2. Cell Death Differ. 2014. PMID: 24786828 Free PMC article.
References
-
- See Cell Signaling Technology website. http://www.cellsignal.com/pdf/9661.pdf.
-
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. - PubMed
-
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, et al. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. - PubMed
-
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. - PubMed
-
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

