Formulation and characterization of echogenic lipid-Pluronic nanobubbles
- PMID: 19957968
- PMCID: PMC3285380
- DOI: 10.1021/mp9001816
Formulation and characterization of echogenic lipid-Pluronic nanobubbles
Abstract
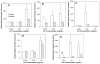
The advent of microbubble contrast agents has enhanced the capabilities of ultrasound as a medical imaging modality and stimulated innovative strategies for ultrasound-mediated drug and gene delivery. While the utilization of microbubbles as carrier vehicles has shown encouraging results in cancer therapy, their applicability has been limited by a large size which typically confines them to the vasculature. To enhance their multifunctional contrast and delivery capacity, it is critical to reduce bubble size to the nanometer range without reducing echogenicity. In this work, we present a novel strategy for formulation of nanosized, echogenic lipid bubbles by incorporating the surfactant Pluronic, a triblock copolymer of ethylene oxide copropylene oxide coethylene oxide into the formulation. Five Pluronics (L31, L61, L81, L64 and P85) with a range of molecular weights (M(w): 1100 to 4600 Da) were incorporated into the lipid shell either before or after lipid film hydration and before addition of perfluorocarbon gas. Results demonstrate that Pluronic-lipid interactions lead to a significantly reduced bubble size. Among the tested formulations, bubbles made with Pluronic L61 were the smallest with a mean hydrodynamic diameter of 207.9 +/- 74.7 nm compared to the 880.9 +/- 127.6 nm control bubbles. Pluronic L81 also significantly reduced bubble size to 406.8 +/- 21.0 nm. We conclude that Pluronic is effective in lipid bubble size control, and Pluronic M(w), hydrophilic-lipophilic balance (HLB), and Pluronic/lipid ratio are critical determinants of the bubble size. Most importantly, our results have shown that although the bubbles are nanosized, their stability and in vitro and in vivo echogenicity are not compromised. The resulting nanobubbles may be better suited for contrast enhanced tumor imaging and subsequent therapeutic delivery.
Figures
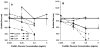
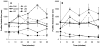
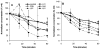




Similar articles
-
Nanobubble ultrasound contrast agents for enhanced delivery of thermal sensitizer to tumors undergoing radiofrequency ablation.Pharm Res. 2014 Jun;31(6):1407-17. doi: 10.1007/s11095-013-1100-x. Epub 2013 Aug 14. Pharm Res. 2014. PMID: 23943542 Free PMC article.
-
Time-intensity-curve Analysis and Tumor Extravasation of Nanobubble Ultrasound Contrast Agents.Ultrasound Med Biol. 2019 Sep;45(9):2502-2514. doi: 10.1016/j.ultrasmedbio.2019.05.025. Epub 2019 Jun 24. Ultrasound Med Biol. 2019. PMID: 31248638 Free PMC article.
-
Effect of Bubble Concentration on the in Vitro and in Vivo Performance of Highly Stable Lipid Shell-Stabilized Micro- and Nanoscale Ultrasound Contrast Agents.Langmuir. 2019 Aug 6;35(31):10192-10202. doi: 10.1021/acs.langmuir.9b00462. Epub 2019 Apr 9. Langmuir. 2019. PMID: 30913884
-
Ultrasound Molecular Imaging of Cancer: Design and Formulation Strategies of Targeted Contrast Agents.Recent Results Cancer Res. 2020;216:319-336. doi: 10.1007/978-3-030-42618-7_9. Recent Results Cancer Res. 2020. PMID: 32594391 Review.
-
Stabilized sulfur hexafluoride microbubbles.2006 Sep 21 [updated 2008 Feb 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Sep 21 [updated 2008 Feb 25]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641961 Free Books & Documents. Review.
Cited by
-
Preparation of multifunctional nanobubbles and their application in bimodal imaging and targeted combination therapy of early pancreatic cancer.Sci Rep. 2021 Mar 18;11(1):6254. doi: 10.1038/s41598-021-82602-9. Sci Rep. 2021. PMID: 33737559 Free PMC article.
-
Improving performance of nanoscale ultrasound contrast agents using N,N-diethylacrylamide stabilization.Nanomedicine. 2017 Jan;13(1):59-67. doi: 10.1016/j.nano.2016.08.020. Epub 2016 Aug 23. Nanomedicine. 2017. PMID: 27565686 Free PMC article.
-
Nanosized Contrast Agents in Ultrasound Molecular Imaging.Front Bioeng Biotechnol. 2021 Nov 29;9:758084. doi: 10.3389/fbioe.2021.758084. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34912789 Free PMC article. Review.
-
Concurrent visual and acoustic tracking of passive and active delivery of nanobubbles to tumors.Theranostics. 2020 Sep 23;10(25):11690-11706. doi: 10.7150/thno.51316. eCollection 2020. Theranostics. 2020. PMID: 33052241 Free PMC article.
-
Differentiation of benign periablational enhancement from residual tumor following radio-frequency ablation using contrast-enhanced ultrasonography in a rat subcutaneous colon cancer model.Ultrasound Med Biol. 2012 Mar;38(3):443-53. doi: 10.1016/j.ultrasmedbio.2011.12.008. Epub 2012 Jan 21. Ultrasound Med Biol. 2012. PMID: 22266229 Free PMC article.
References
-
- Wheatley MA, Schrope B, Shen P. Contrast agents for diagnostic ultrasound: development and evaluation of polymer-coated microbubbles. Biomaterials. 1990;11(9):713–7. - PubMed
-
- Schneider M, et al. BR1: a new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30(8):451–7. - PubMed
-
- Bjerknes K, et al. Air-filled polymeric microcapsules from emulsions containing different organic phases. J Microencapsulation. 2001;18(2):159–71. - PubMed
-
- Cavalieri F, et al. Stable polymeric microballoons as multifunctional device for biomedical uses: synthesis and characterization. Langmuir. 2005;21(19):8758–64. - PubMed
-
- Feinstein SB, et al. Safety and efficacy of a new transpulmonary ultrasound contrast agent: initial multicenter clinical results. J Am Coll Cardiol. 1990;16(2):316–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

