Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif
- PMID: 19956681
- PMCID: PMC2778012
- DOI: 10.1371/journal.pone.0008045
Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif
Abstract
Background: Rho family GTPases are critical regulators of the cytoskeleton and affect cell migration, cell-cell adhesion, and cell-matrix adhesion. As with all GTPases, their activity is determined by their guanine nucleotide-bound state. Understanding how Rho proteins are activated and inactivated has largely focused on regulatory proteins such as guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). However, recent in vitro studies have indicated that GTPases may also be directly regulated by redox agents. We hypothesized that this redox-based mechanism occurs in cells and affects cytoskeletal dynamics, and in this report we conclude this is indeed a novel mechanism of regulating the GTPase RhoA.
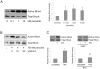
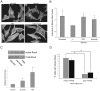
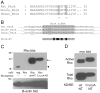
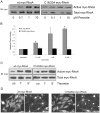
Methodology/principal findings: In this report, we show that RhoA can be directly activated by reactive oxygen species (ROS) in cells, and that this requires two critical cysteine residues located in a unique redox-sensitive motif within the phosphoryl binding loop. First, we show that ROS can reversibly activate RhoA and induce stress fiber formation, a well characterized readout of RhoA activity. To determine the role of cysteine residues in this mechanism of regulation, we generated cysteine to alanine RhoA mutants. Mutation of these cysteines abolishes ROS-mediated activation and stress fiber formation, indicating that these residues are critical for redox-regulation of RhoA. Importantly, these mutants maintain the ability to be activated by GEFs.
Conclusions/significance: Our findings identify a novel mechanism for the regulation of RhoA in cells by ROS, which is independent of classical regulatory proteins. This mechanism of regulation may be particularly relevant in pathological conditions where ROS are generated and the cellular redox-balance altered, such as in asthma and ischemia-reperfusion injury.
Conflict of interest statement
Figures
Similar articles
-
XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC.J Biol Chem. 2002 Nov 8;277(45):42964-72. doi: 10.1074/jbc.M207401200. Epub 2002 Sep 6. J Biol Chem. 2002. PMID: 12221096
-
G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production.Circ Res. 2003 Oct 31;93(9):848-56. doi: 10.1161/01.RES.0000097607.14733.0C. Epub 2003 Sep 25. Circ Res. 2003. PMID: 14512443
-
The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton.Mol Cell Biol. 1996 Oct;16(10):5313-27. doi: 10.1128/MCB.16.10.5313. Mol Cell Biol. 1996. PMID: 8816443 Free PMC article.
-
Regulation of RhoA activity by adhesion molecules and mechanotransduction.Curr Mol Med. 2014 Feb;14(2):199-208. doi: 10.2174/1566524014666140128104541. Curr Mol Med. 2014. PMID: 24467208 Free PMC article. Review.
-
Rho GTPases: Non-canonical regulation by cysteine oxidation.Bioessays. 2022 Feb;44(2):e2100152. doi: 10.1002/bies.202100152. Epub 2021 Dec 10. Bioessays. 2022. PMID: 34889471 Review.
Cited by
-
Molecular Crosstalk between Integrins and Cadherins: Do Reactive Oxygen Species Set the Talk?J Signal Transduct. 2012;2012:807682. doi: 10.1155/2012/807682. Epub 2011 Dec 13. J Signal Transduct. 2012. PMID: 22203898 Free PMC article.
-
Regulation of Ras proteins by reactive nitrogen species.Free Radic Biol Med. 2011 Aug 1;51(3):565-75. doi: 10.1016/j.freeradbiomed.2011.05.003. Epub 2011 May 8. Free Radic Biol Med. 2011. PMID: 21616138 Free PMC article. Review.
-
Rho protein crosstalk: another social network?Trends Cell Biol. 2011 Dec;21(12):718-26. doi: 10.1016/j.tcb.2011.08.002. Epub 2011 Sep 15. Trends Cell Biol. 2011. PMID: 21924908 Free PMC article. Review.
-
Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase.Am J Physiol Lung Cell Mol Physiol. 2011 Dec;301(6):L847-57. doi: 10.1152/ajplung.00178.2011. Epub 2011 Oct 7. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21984567 Free PMC article.
-
Arecoline Increases Glycolysis and Modulates pH Regulator Expression in HA22T/VGH Hepatoma Cells, Leading to Increase of Intracellular Ca2+, Reactive Oxygen Species, and Anoikis.J Cancer. 2017 Sep 15;8(16):3173-3182. doi: 10.7150/jca.20523. eCollection 2017. J Cancer. 2017. PMID: 29158789 Free PMC article.
References
-
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. - PubMed
-
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. - PubMed
-
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. - PubMed
-
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. - PubMed
-
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL080166/HL/NHLBI NIH HHS/United States
- 1F30HL094063/HL/NHLBI NIH HHS/United States
- F30 HL094063-01A1/HL/NHLBI NIH HHS/United States
- F30 HL094063/HL/NHLBI NIH HHS/United States
- R01 GM029860/GM/NIGMS NIH HHS/United States
- R01 CA089614-06A1/CA/NCI NIH HHS/United States
- T32 GM008719/GM/NIGMS NIH HHS/United States
- R01 CA089614/CA/NCI NIH HHS/United States
- HL045100/HL/NHLBI NIH HHS/United States
- GM029860/GM/NIGMS NIH HHS/United States
- P01 HL045100/HL/NHLBI NIH HHS/United States
- P01 HL080166/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

