Nucleolin is required for efficient nuclear egress of herpes simplex virus type 1 nucleocapsids
- PMID: 19955312
- PMCID: PMC2812367
- DOI: 10.1128/JVI.02007-09
Nucleolin is required for efficient nuclear egress of herpes simplex virus type 1 nucleocapsids
Abstract
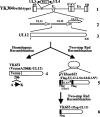
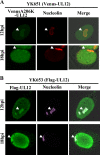
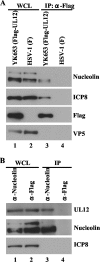
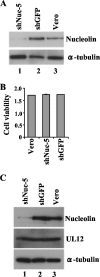
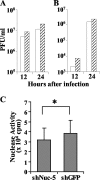
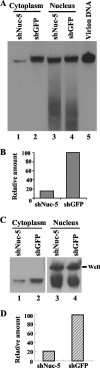
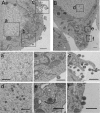
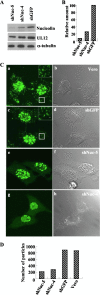
Herpesvirus nucleocapsids assemble in the nucleus and must cross the nuclear membrane for final assembly and maturation to form infectious progeny virions in the cytoplasm. It has been proposed that nucleocapsids enter the perinuclear space by budding through the inner nuclear membrane, and these enveloped nucleocapsids then fuse with the outer nuclear membrane to enter the cytoplasm. Little is known about the mechanism(s) for nuclear egress of herpesvirus nucleocapsids and, in particular, which, if any, cellular proteins are involved in the nuclear egress pathway. UL12 is an alkaline nuclease encoded by herpes simplex virus type 1 (HSV-1) and has been suggested to be involved in viral DNA maturation and nuclear egress of nucleocapsids. Using a live-cell imaging system to study cells infected by a recombinant HSV-1 expressing UL12 fused to a fluorescent protein, we observed the previously unreported nucleolar localization of UL12 in live infected cells and, using coimmunoprecipitation analyses, showed that UL12 formed a complex with nucleolin, a nucleolus marker, in infected cells. Knockdown of nucleolin in HSV-1-infected cells reduced capsid accumulation, as well as the amount of viral DNA resistant to staphylococcal nuclease in the cytoplasm, which represented encapsidated viral DNA, but had little effect on these viral components in the nucleus. These results indicated that nucleolin is a cellular factor required for efficient nuclear egress of HSV-1 nucleocapsids in infected cells.
Figures

Similar articles
-
Identification of the Capsid Binding Site in the Herpes Simplex Virus 1 Nuclear Egress Complex and Its Role in Viral Primary Envelopment and Replication.J Virol. 2019 Oct 15;93(21):e01290-19. doi: 10.1128/JVI.01290-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31391274 Free PMC article.
-
Herpes simplex virus 1 UL47 interacts with viral nuclear egress factors UL31, UL34, and Us3 and regulates viral nuclear egress.J Virol. 2014 May;88(9):4657-67. doi: 10.1128/JVI.00137-14. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522907 Free PMC article.
-
Role of Host Cell p32 in Herpes Simplex Virus 1 De-Envelopment during Viral Nuclear Egress.J Virol. 2015 Sep;89(17):8982-98. doi: 10.1128/JVI.01220-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085152 Free PMC article.
-
Host and Viral Factors Involved in Nuclear Egress of Herpes Simplex Virus 1.Viruses. 2021 Apr 25;13(5):754. doi: 10.3390/v13050754. Viruses. 2021. PMID: 33923040 Free PMC article. Review.
-
Virus Assembly and Egress of HSV.Adv Exp Med Biol. 2018;1045:23-44. doi: 10.1007/978-981-10-7230-7_2. Adv Exp Med Biol. 2018. PMID: 29896661 Review.
Cited by
-
Nucleolin down-regulation is involved in ADP-induced cell cycle arrest in S phase and cell apoptosis in vascular endothelial cells.PLoS One. 2014 Oct 7;9(10):e110101. doi: 10.1371/journal.pone.0110101. eCollection 2014. PLoS One. 2014. PMID: 25290311 Free PMC article.
-
A CRISPR-based rapid DNA repositioning strategy and the early intranuclear life of HSV-1.Elife. 2023 Sep 13;12:e85412. doi: 10.7554/eLife.85412. Elife. 2023. PMID: 37702383 Free PMC article.
-
Seneca Valley Virus 3Cpro Mediates Cleavage and Redistribution of Nucleolin To Facilitate Viral Replication.Microbiol Spectr. 2022 Apr 27;10(2):e0030422. doi: 10.1128/spectrum.00304-22. Epub 2022 Mar 31. Microbiol Spectr. 2022. PMID: 35357201 Free PMC article.
-
Nucleolin Promotes IRES-Driven Translation of Foot-and-Mouth Disease Virus by Supporting the Assembly of Translation Initiation Complexes.J Virol. 2021 Jun 10;95(13):e0023821. doi: 10.1128/JVI.00238-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 33853964 Free PMC article.
-
Nucleolin interacts with the rabbit hemorrhagic disease virus replicase RdRp, nonstructural proteins p16 and p23, playing a role in virus replication.Virol Sin. 2022 Feb;37(1):48-59. doi: 10.1016/j.virs.2022.01.004. Epub 2022 Jan 12. Virol Sin. 2022. PMID: 35234629 Free PMC article.
References
-
- De, A., S. L. Donahue, A. Tabah, N. E. Castro, N. Mraz, J. L. Cruise, and C. Campbell. 2006. A novel interaction of nucleolin with Rad51. Biochem. Biophys. Res. Commun. 344:206-213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

