Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia
- PMID: 19948968
- PMCID: PMC2787122
- DOI: 10.1073/pnas.0911921106
Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia
Abstract
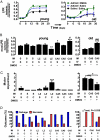
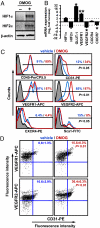
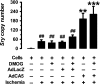
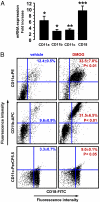
Ischemia induces the production of angiogenic cytokines and the homing of bone-marrow-derived angiogenic cells (BMDACs), but these adaptive responses become impaired with aging because of reduced expression of hypoxia-inducible factor (HIF)-1alpha. In this study, we analyzed the effect of augmenting HIF-1alpha levels in ischemic limb by intramuscular injection of AdCA5, an adenovirus encoding a constitutively active form of HIF-1alpha, and intravenous administration of BMDACs that were cultured in the presence of the prolyl-4-hydroxylase inhibitor dimethyloxalylglycine (DMOG) to induce HIF-1 expression. The combined therapy increased perfusion, motor function, and limb salvage in old mice subjected to femoral artery ligation. Homing of BMDACs to the ischemic limb was dramatically enhanced by intramuscular AdCA5 administration. DMOG treatment of BMDACs increased cell surface expression of beta(2) integrins, which mediated increased adherence of BMDACs to endothelial cells. The effect of DMOG was abolished by coadministration of the HIF-1 inhibitor digoxin or by preincubation with a beta(2) integrin-blocking antibody. Transduction of BMDACs with lentivirus LvCA5 induced effects similar to DMOG treatment. Thus, HIF-1alpha gene therapy increases homing of BMDACs to ischemic muscle, whereas HIF-1 induction in BMDACs enhances their adhesion to vascular endothelium, leading to synergistic effects of combined therapy on tissue perfusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Combination of HIF-1α gene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice.Gene Ther. 2013 Nov;20(11):1070-6. doi: 10.1038/gt.2013.32. Epub 2013 Jun 20. Gene Ther. 2013. PMID: 23784441
-
Systemic preconditioning by a prolyl hydroxylase inhibitor promotes prevention of skin flap necrosis via HIF-1-induced bone marrow-derived cells.PLoS One. 2012;7(8):e42964. doi: 10.1371/journal.pone.0042964. Epub 2012 Aug 7. PLoS One. 2012. PMID: 22880134 Free PMC article.
-
Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia.Cardiovasc Res. 2005 Oct 1;68(1):144-54. doi: 10.1016/j.cardiores.2005.05.002. Cardiovasc Res. 2005. PMID: 15921668
-
In vivo electroporation of constitutively expressed HIF-1α plasmid DNA improves neovascularization in a mouse model of limb ischemia.J Vasc Surg. 2014 Mar;59(3):786-93. doi: 10.1016/j.jvs.2013.04.043. Epub 2013 Jul 11. J Vasc Surg. 2014. PMID: 23850058 Free PMC article.
-
Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization.J Investig Med. 2016 Feb;64(2):361-3. doi: 10.1097/JIM.0000000000000206. Epub 2016 Jan 11. J Investig Med. 2016. PMID: 25955799 Free PMC article. Review.
Cited by
-
Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells.J Cell Biochem. 2016 Feb;117(2):267-78. doi: 10.1002/jcb.25283. J Cell Biochem. 2016. PMID: 26206147 Free PMC article. Review.
-
Akt/hypoxia-inducible factor-1α signaling deficiency compromises skin wound healing in a type 1 diabetes mouse model.Exp Ther Med. 2015 Jun;9(6):2141-2146. doi: 10.3892/etm.2015.2394. Epub 2015 Mar 30. Exp Ther Med. 2015. PMID: 26136949 Free PMC article.
-
From bench to bedside: review of gene and cell-based therapies and the slow advancement into phase 3 clinical trials, with a focus on Aastrom's Ixmyelocel-T.Stem Cell Rev Rep. 2013 Jun;9(3):373-83. doi: 10.1007/s12015-013-9431-x. Stem Cell Rev Rep. 2013. PMID: 23456574 Free PMC article. Review.
-
Endothelial progenitor cells derived from Wharton's jelly of the umbilical cord reduces ischemia-induced hind limb injury in diabetic mice by inducing HIF-1α/IL-8 expression.Stem Cells Dev. 2013 May 1;22(9):1408-18. doi: 10.1089/scd.2012.0445. Epub 2013 Feb 15. Stem Cells Dev. 2013. PMID: 23252631 Free PMC article.
-
Ultrahigh sensitive optical microangiography reveals depth-resolved microcirculation and its longitudinal response to prolonged ischemic event within skeletal muscles in mice.J Biomed Opt. 2011 Aug;16(8):086004. doi: 10.1117/1.3606565. J Biomed Opt. 2011. PMID: 21895316 Free PMC article.
References
-
- Hirsch AT, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease. Circulation. 2006;113:e463–e654. - PubMed
-
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part II: Cell-based therapies. Circulation. 2004;109:2692–2697. - PubMed
-
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: Angiogenic cytokines. Circulation. 2004;109:2487–2491. - PubMed
-
- Bosch-Marcé M, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. - PubMed
-
- Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

