Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo
- PMID: 19948826
- PMCID: PMC2797907
- DOI: 10.2353/ajpath.2010.080642
Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo
Abstract
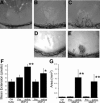
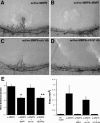
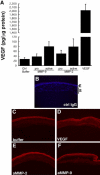
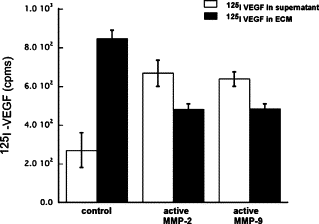
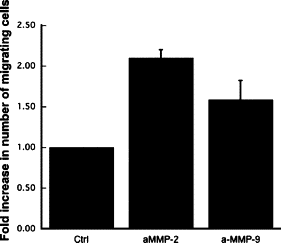
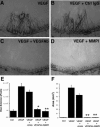
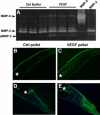
Matrix metalloproteinases (MMPs), a specialized group of enzymes capable of proteolytically degrading extracellular matrix proteins, have been postulated to play an important role in angiogenesis. It has been suggested that MMPs can regulate neovascularization using mechanisms other than simple remodeling of the capillary basement membrane. To determine the interplay between vascular endothelial growth factor (VEGF) and MMPs, we investigated the induction of angiogenesis by recombinant active MMPs and VEGF in vivo. Using a rat corneal micropocket in vivo angiogenesis assay, we observed that the active form of MMP-9 could induce neovascularization in vivo when compared with the pro- form of the enzyme as a control. This angiogenic response could be inhibited by neutralizing VEGF antibody, which suggests that MMPs acts upstream of VEGF. Additional in vitro studies using extracellular matrix loaded with radiolabeled VEGF determined that active MMPs can enzymatically release sequestered VEGF. Interestingly, in vivo angiogenesis induced by VEGF could be inhibited by MMP inhibitors, indicating that MMPs also act downstream of VEGF. In addition, inflammation plays an important role in the induction of angiogenesis mediated by both VEGF and MMPs. Our results suggest that MMPs act both upstream and downstream of VEGF and imply that potential combination therapies of VEGF and MMP inhibitors may be a useful therapeutic approach in diseases of pathological neovascularization.
Figures

Similar articles
-
Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis.Cardiovasc Res. 2008 May 1;78(2):356-65. doi: 10.1093/cvr/cvm111. Epub 2007 Dec 18. Cardiovasc Res. 2008. PMID: 18093986
-
Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation.Molecules. 2014 Aug 13;19(8):12150-72. doi: 10.3390/molecules190812150. Molecules. 2014. PMID: 25123184 Free PMC article.
-
Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis.Basic Res Cardiol. 2006 May;101(3):204-13. doi: 10.1007/s00395-005-0581-0. Epub 2005 Dec 23. Basic Res Cardiol. 2006. PMID: 16369727 Free PMC article.
-
Regulation of matrix metalloproteinases 2 and 9 in corneal neovascularization.Chem Biol Drug Des. 2020 May;95(5):485-492. doi: 10.1111/cbdd.13529. Epub 2020 Feb 16. Chem Biol Drug Des. 2020. PMID: 31002472 Review.
-
Matrix metalloproteinases: new routes to the use of MT1-MMP as a therapeutic target in angiogenesis-related disease.Curr Pharm Des. 2007;13(17):1787-802. doi: 10.2174/138161207780831284. Curr Pharm Des. 2007. PMID: 17584108 Review.
Cited by
-
Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature.Matrix Biol. 2015 May-Jul;44-46:94-112. doi: 10.1016/j.matbio.2015.04.004. Epub 2015 Apr 22. Matrix Biol. 2015. PMID: 25912949 Free PMC article. Review.
-
Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer.FASEB J. 2013 Jan;27(1):45-50. doi: 10.1096/fj.12-211730. Epub 2012 Sep 14. FASEB J. 2013. PMID: 22982376 Free PMC article.
-
Activation of the GPR35 pathway drives angiogenesis in the tumour microenvironment.Gut. 2022 Mar;71(3):509-520. doi: 10.1136/gutjnl-2020-323363. Epub 2021 Mar 23. Gut. 2022. PMID: 33758004 Free PMC article.
-
Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres.Biomaterials. 2012 Apr;33(12):3475-84. doi: 10.1016/j.biomaterials.2012.01.032. Epub 2012 Feb 7. Biomaterials. 2012. PMID: 22322198 Free PMC article.
-
Altered angiogenesis in caveolin-1 gene-deficient mice is restored by ablation of endothelial nitric oxide synthase.Am J Pathol. 2012 Apr;180(4):1702-14. doi: 10.1016/j.ajpath.2011.12.018. Epub 2012 Feb 7. Am J Pathol. 2012. PMID: 22322296 Free PMC article.
References
-
- Pepper MS, Montesano R, Mandriota SJ, Orci L, Vassalli JD. Angiogenesis: a paradigm for balanced extracellular proteolysis during cell migration and morphogenesis. Enzyme Protein. 1996;49:138–162. - PubMed
-
- Matrisian LM. The matrix degrading metalloproteinases. Bioessays. 1992;14:455–463. - PubMed
-
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

