MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts
- PMID: 19948485
- PMCID: PMC2779245
- DOI: 10.1083/jcb.200904049
MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts
Abstract
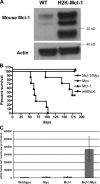
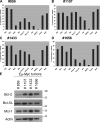
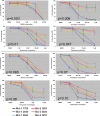
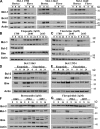
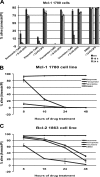
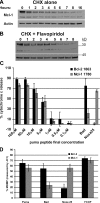
Myeloid cell leukemia sequence 1 (MCL-1) and B cell leukemia/lymphoma 2 (BCL-2) are anti-apoptotic proteins in the BCL-2 protein family often expressed in cancer. To compare the function of MCL-1 and BCL-2 in maintaining cancer survival, we constructed complementary mouse leukemia models based on Emu-Myc expression in which either BCL-2 or MCL-1 are required for leukemia maintenance. We show that the principal anti-apoptotic mechanism of both BCL-2 and MCL-1 in these leukemias is to sequester pro-death BH3-only proteins rather than BAX and BAK. We find that the MCL-1-dependent leukemias are more sensitive to a wide range of chemotherapeutic agents acting by disparate mechanisms. In common across these varied treatments is that MCL-1 protein levels rapidly decrease in a proteosome-dependent fashion, whereas those of BCL-2 are stable. We demonstrate for the first time that two anti-apoptotic proteins can enable tumorigenesis equally well, but nonetheless differ in their influence on chemosensitivity.
Figures




Similar articles
-
Bim must be able to engage all pro-survival Bcl-2 family members for efficient tumor suppression.Oncogene. 2012 Jul 12;31(28):3392-6. doi: 10.1038/onc.2011.508. Epub 2011 Nov 14. Oncogene. 2012. PMID: 22081075 Free PMC article.
-
Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.Nat Cell Biol. 2006 Dec;8(12):1348-58. doi: 10.1038/ncb1499. Epub 2006 Nov 19. Nat Cell Biol. 2006. PMID: 17115033
-
Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance.Blood. 2010 Oct 28;116(17):3197-207. doi: 10.1182/blood-2010-04-281071. Epub 2010 Jul 14. Blood. 2010. PMID: 20631380 Free PMC article.
-
Bcl-2 family proteins in breast development and cancer: could Mcl-1 targeting overcome therapeutic resistance?Oncotarget. 2015 Feb 28;6(6):3519-30. doi: 10.18632/oncotarget.2792. Oncotarget. 2015. PMID: 25784482 Free PMC article. Review.
-
Mcl-1 is a potential therapeutic target in multiple types of cancer.Cell Mol Life Sci. 2009 Apr;66(8):1326-36. doi: 10.1007/s00018-008-8637-6. Cell Mol Life Sci. 2009. PMID: 19099185 Free PMC article. Review.
Cited by
-
Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia.Cancer Discov. 2014 Mar;4(3):362-75. doi: 10.1158/2159-8290.CD-13-0609. Epub 2013 Dec 17. Cancer Discov. 2014. PMID: 24346116 Free PMC article.
-
The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy.Cell Death Differ. 2011 Sep;18(9):1414-24. doi: 10.1038/cdd.2011.17. Epub 2011 Mar 18. Cell Death Differ. 2011. PMID: 21415859 Free PMC article. Review.
-
BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions.Cancer Lett. 2013 May 28;332(2):202-5. doi: 10.1016/j.canlet.2011.12.021. Epub 2012 Jan 8. Cancer Lett. 2013. PMID: 22230093 Free PMC article. Review.
-
Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML.Cell. 2012 Oct 12;151(2):344-55. doi: 10.1016/j.cell.2012.08.038. Cell. 2012. PMID: 23063124 Free PMC article.
-
Genomic profiles in B cell lymphoma.Int J Hematol. 2010 Sep;92(2):238-45. doi: 10.1007/s12185-010-0662-1. Epub 2010 Aug 27. Int J Hematol. 2010. PMID: 20799004 Review.
References
-
- Brunelle J.K., Santore M.T., Budinger G.R., Tang Y., Barrett T.A., Zong W.X., Kandel E., Keith B., Simon M.C., Thompson C.B., et al. 2004. c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1. J. Biol. Chem. 279:4305–4312 10.1074/jbc.M312241200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

