Adhesion and migration, the diverse functions of the laminin alpha3 subunit
- PMID: 19945619
- PMCID: PMC2814596
- DOI: 10.1016/j.det.2009.10.009
Adhesion and migration, the diverse functions of the laminin alpha3 subunit
Abstract
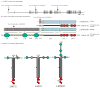
The laminins are a secreted family of heterotrimeric molecules essential for basement membrane formation, structure, and function. It is now well established that the alpha3 subunit of laminins-332, -321, and -311 plays an important role in mediating epidermal-dermal integrity and is essential for the skin to withstand mechanical stresses. These laminins also regulate cell migration and mechanosignal transduction. This article provides an overview of the gene, transcripts, and protein structures of laminin alpha3. Also discussed are the proposed functions for the alpha3 subunit-containing laminins.
Figures
Similar articles
-
The functions of exogenous and endogenous laminin-5 on corneal epithelial cells.Exp Eye Res. 2000 Jul;71(1):69-79. doi: 10.1006/exer.2000.0857. Exp Eye Res. 2000. PMID: 10880277
-
Plasmin induces degradation and dysfunction of laminin 332 (laminin 5) and impaired assembly of basement membrane at the dermal-epidermal junction.Br J Dermatol. 2008 Jul;159(1):49-60. doi: 10.1111/j.1365-2133.2008.08576.x. Epub 2008 Jul 1. Br J Dermatol. 2008. PMID: 18460030
-
Initiation of skin basement membrane formation at the epidermo-dermal interface involves assembly of laminins through binding to cell membrane receptors.J Cell Sci. 1998 Jul 30;111 ( Pt 14):1929-40. doi: 10.1242/jcs.111.14.1929. J Cell Sci. 1998. PMID: 9645941
-
Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction.Exp Dermatol. 2021 Jan;30(1):17-24. doi: 10.1111/exd.14239. Epub 2020 Dec 11. Exp Dermatol. 2021. PMID: 33205478 Review.
-
The importance of laminin 5 in the dermal-epidermal basement membrane.J Dermatol Sci. 2000 Dec;24 Suppl 1:S51-9. doi: 10.1016/s0923-1811(00)00142-0. J Dermatol Sci. 2000. PMID: 11137397 Review.
Cited by
-
Xeno-Free Strategies for Safe Human Mesenchymal Stem/Stromal Cell Expansion: Supplements and Coatings.Stem Cells Int. 2017;2017:6597815. doi: 10.1155/2017/6597815. Epub 2017 Oct 11. Stem Cells Int. 2017. PMID: 29158740 Free PMC article. Review.
-
Changes in laminin chain expression in pre- and postnatal rat pituitary gland.Acta Histochem Cytochem. 2014;47(5):231-7. doi: 10.1267/ahc.14031. Epub 2014 Oct 23. Acta Histochem Cytochem. 2014. PMID: 25861129 Free PMC article.
-
Identification of AGR2 Gene-Specific Expression Patterns Associated with Epithelial-Mesenchymal Transition.Int J Mol Sci. 2022 Sep 16;23(18):10845. doi: 10.3390/ijms231810845. Int J Mol Sci. 2022. PMID: 36142758 Free PMC article.
-
Type VI collagen promotes lung epithelial cell spreading and wound-closure.PLoS One. 2018 Dec 14;13(12):e0209095. doi: 10.1371/journal.pone.0209095. eCollection 2018. PLoS One. 2018. PMID: 30550606 Free PMC article.
-
Novel variants in LAMA3 and COL7A1 and recurrent variant in KRT5 underlying epidermolysis bullosa in five Chinese families.Front Med. 2022 Oct;16(5):808-814. doi: 10.1007/s11684-021-0878-x. Epub 2022 Mar 21. Front Med. 2022. PMID: 35314946

