Regulation of heat shock protein 60 and 72 expression in the failing heart
- PMID: 19945465
- PMCID: PMC2814075
- DOI: 10.1016/j.yjmcc.2009.11.009
Regulation of heat shock protein 60 and 72 expression in the failing heart
Abstract
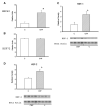
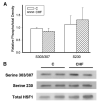
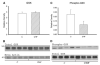
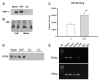
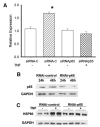
Heart failure, a progressive, fatal disease of the heart muscle, is a state of chronic inflammation and injury. Heat shock protein (HSP) 72, a ubiquitous protective protein that is well-established as cardioprotective, is not increased in heart failure. In contrast, HSP60 levels are doubled in the failing heart. We hypothesized that HSF-1 is not activated in heart failure and that the increased expression of HSP60 was driven by NFkappaB activation. To test this hypothesis, we measured levels of heat shock factor (HSF) -1 and -2, the transcription factors controlling HSP expression, which were increased in heart failure. There was no increased phosphorylation of serine 230 or serine 303/307 in HSF-1, which are thought to regulate its activity; EMSA showed no increase in HSF binding activity with heart failure. Nonetheless, mRNA was increased for HSP60, but not HSP72. In contrast to HSF, NFkappaB activity was increased in heart failure. HSP60, but not HSP72, contained NFkappaB binding elements. ChIP assay demonstrated increased binding of NFkappaB to both of the NFkappaB binding elements in the heart failure HSP60 gene. TNFalpha treatment was used to test the role of NFkappaB activation in HSP60 expression in a cardiac cell line. TNFalpha increased HSP60 expression, and this could be prevented by pretreatment with siRNA inhibiting p65 expression. In conclusion, HSP72 is not increased in heart failure because HSF activity is not changed; increased expression of HSP60 may be driven by NFkappaB activation.
Published by Elsevier Ltd.
Figures

Similar articles
-
Estrogen, heat shock proteins, and NFkappaB in human vascular endothelium.Arterioscler Thromb Vasc Biol. 2004 Sep;24(9):1628-33. doi: 10.1161/01.ATV.0000137188.76195.fb. Epub 2004 Jul 1. Arterioscler Thromb Vasc Biol. 2004. PMID: 15231513
-
Coordinated transcriptional regulation of Hspa1a gene by multiple transcription factors: crucial roles for HSF-1, NF-Y, NF-κB, and CREB.J Mol Biol. 2014 Jan 9;426(1):116-35. doi: 10.1016/j.jmb.2013.09.008. Epub 2013 Sep 14. J Mol Biol. 2014. PMID: 24041570
-
Previous heat shock facilitates the glutamine-induced expression of heat-shock protein 72 in septic liver.Nutrition. 2007 Jul-Aug;23(7-8):582-8. doi: 10.1016/j.nut.2007.04.013. Nutrition. 2007. PMID: 17616344
-
Heat shock proteins with an emphasis on HSP 60.Mol Biol Rep. 2021 Oct;48(10):6959-6969. doi: 10.1007/s11033-021-06676-4. Epub 2021 Sep 8. Mol Biol Rep. 2021. PMID: 34498161 Review.
-
Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story.Med Res Rev. 2021 Jan;41(1):29-71. doi: 10.1002/med.21723. Epub 2020 Aug 17. Med Res Rev. 2021. PMID: 32808366 Free PMC article. Review.
Cited by
-
Potential of Natural Products in the Treatment of Glioma: Focus on Molecular Mechanisms.Cell Biochem Biophys. 2024 Dec;82(4):3157-3208. doi: 10.1007/s12013-024-01447-x. Epub 2024 Aug 16. Cell Biochem Biophys. 2024. PMID: 39150676 Review.
-
Pathobiological Mechanisms of Endothelial Dysfunction Induced by tert-Butyl Hydroperoxide via Apoptosis, Necrosis and Senescence in a Rat Model.Int J Med Sci. 2020 Feb 4;17(3):368-382. doi: 10.7150/ijms.40255. eCollection 2020. Int J Med Sci. 2020. PMID: 32132872 Free PMC article.
-
HSP60 mediates the neuroprotective effects of curcumin by suppressing microglial activation.Exp Ther Med. 2016 Aug;12(2):823-828. doi: 10.3892/etm.2016.3413. Epub 2016 Jun 1. Exp Ther Med. 2016. PMID: 27446282 Free PMC article.
-
Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4.J Biol Chem. 2011 Sep 9;286(36):31308-19. doi: 10.1074/jbc.M111.246124. Epub 2011 Jul 20. J Biol Chem. 2011. PMID: 21775438 Free PMC article.
-
Neuroprotective effect of heat shock protein 60 on matrine-suppressed microglial activation.Exp Ther Med. 2017 Aug;14(2):1832-1836. doi: 10.3892/etm.2017.4691. Epub 2017 Jun 27. Exp Ther Med. 2017. PMID: 28781634 Free PMC article.
References
-
- Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure: focus on cardiac hypertrophy, inflammation, angiogenesis, and apoptosis. J Am Coll Cardiol. 2006 Oct 27;48(9SupplA):A56–66.
-
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. JNCI Cancer Spectr. 2000 Oct 4;92(19):1564–72. - PubMed
-
- Beckmann RP, Mizzen LA, Welch WJ. Interaction of HSP 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–4. - PubMed
-
- Beck SC, De Maio A. Stabilization of protein synthesis of thermotolerant cells during heat shock: association of heat shock protein-72 with ribosomal subunits of polysomes. J Biol Chem. 1994;269:21803–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

