The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163
- PMID: 19939927
- PMCID: PMC2812361
- DOI: 10.1128/JVI.01774-09
The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163
Abstract
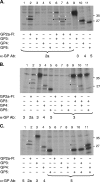
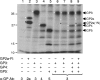
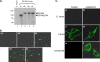
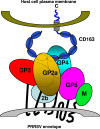
Porcine reproductive and respiratory syndrome virus (PRRSV) contains the major glycoprotein, GP5, as well as three other minor glycoproteins, namely, GP2a, GP3, and GP4, on the virion envelope, all of which are required for generation of infectious virions. To study their interactions with each other and with the cellular receptor for PRRSV, we have cloned each of the viral glycoproteins and CD163 receptor in expression vectors and examined their expression and interaction with each other in transfected cells by coimmunoprecipitation (co-IP) assay using monospecific antibodies. Our results show that a strong interaction exists between the GP4 and GP5 proteins, although weak interactions among the other minor envelope glycoproteins and GP5 have been detected. Both GP2a and GP4 proteins were found to interact with all the other GPs, resulting in the formation of multiprotein complex. Our results further show that the GP2a and GP4 proteins also specifically interact with the CD163 molecule. The carboxy-terminal 223 residues of the CD163 molecule are not required for interactions with either the GP2a or the GP4 protein, although these residues are required for conferring susceptibility to PRRSV infection in BHK-21 cells. Overall, we conclude that the GP4 protein is critical for mediating interglycoprotein interactions and, along with GP2a, serves as the viral attachment protein that is responsible for mediating interactions with CD163 for virus entry into susceptible host cell.
Figures
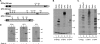
Similar articles
-
Glycosyl-phosphatidylinositol (GPI)-anchored membrane association of the porcine reproductive and respiratory syndrome virus GP4 glycoprotein and its co-localization with CD163 in lipid rafts.Virology. 2012 Mar 1;424(1):18-32. doi: 10.1016/j.virol.2011.12.009. Epub 2012 Jan 4. Virology. 2012. PMID: 22222209 Free PMC article.
-
Phages bearing specific peptides with affinity for porcine reproductive and respiratory syndrome virus GP4 protein prevent cell penetration of the virus.Vet Microbiol. 2018 Oct;224:43-49. doi: 10.1016/j.vetmic.2018.08.027. Epub 2018 Aug 29. Vet Microbiol. 2018. PMID: 30269789
-
Glycosylation of minor envelope glycoproteins of porcine reproductive and respiratory syndrome virus in infectious virus recovery, receptor interaction, and immune response.Virology. 2011 Feb 20;410(2):385-94. doi: 10.1016/j.virol.2010.12.002. Epub 2010 Dec 30. Virology. 2011. PMID: 21195444
-
A brief review of CD163 and its role in PRRSV infection.Virus Res. 2010 Dec;154(1-2):98-103. doi: 10.1016/j.virusres.2010.07.018. Epub 2010 Jul 23. Virus Res. 2010. PMID: 20655964 Review.
-
Gene editing as applied to prevention of reproductive porcine reproductive and respiratory syndrome.Mol Reprod Dev. 2017 Sep;84(9):926-933. doi: 10.1002/mrd.22811. Epub 2017 Jun 8. Mol Reprod Dev. 2017. PMID: 28390179 Review.
Cited by
-
Linear epitopes of PRRSV-1 envelope proteins ectodomains are not correlated with broad neutralization.Porcine Health Manag. 2024 Oct 21;10(1):44. doi: 10.1186/s40813-024-00393-7. Porcine Health Manag. 2024. PMID: 39434120 Free PMC article.
-
Using Alphafold2 to Predict the Structure of the Gp5/M Dimer of Porcine Respiratory and Reproductive Syndrome Virus.Int J Mol Sci. 2022 Oct 30;23(21):13209. doi: 10.3390/ijms232113209. Int J Mol Sci. 2022. PMID: 36361998 Free PMC article.
-
Challenges for Porcine Reproductive and Respiratory Syndrome (PRRS) Vaccine Design: Reviewing Virus Glycoprotein Interactions with CD163 and Targets of Virus Neutralization.Vet Sci. 2019 Jan 17;6(1):9. doi: 10.3390/vetsci6010009. Vet Sci. 2019. PMID: 30658381 Free PMC article. Review.
-
Reappraising host cellular factors involved in attachment and entry to develop antiviral strategies against porcine reproductive and respiratory syndrome virus.Front Microbiol. 2022 Jul 26;13:975610. doi: 10.3389/fmicb.2022.975610. eCollection 2022. Front Microbiol. 2022. PMID: 35958155 Free PMC article. Review.
-
Packaging of porcine reproductive and respiratory syndrome virus replicon RNA by a stable cell line expressing its nucleocapsid protein.J Microbiol. 2011 Jun;49(3):516-23. doi: 10.1007/s12275-011-1280-1. Epub 2011 Jun 30. J Microbiol. 2011. PMID: 21717343 Free PMC article.
References
-
- Balasuriya, U. B., and N. J. MacLachlan. 2004. The immune response to equine arteritis virus: potential lessons for other arteriviruses. Vet. Immunol. Immunopathol. 102:107-129. - PubMed
-
- Ben-Dor, S., N. Esterman, E. Rubin, and N. Sharon. 2004. Biases and complex patterns in the residues flanking protein N-glycosylation sites. Glycobiology 14:95-101. - PubMed
-
- Bolt, G., C. Kristensen, and T. D. Steenstrup. 2005. Posttranslational N-glycosylation takes place during the normal processing of human coagulation factor VII. Glycobiology 15:541-547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

