Protein kinase C: poised to signal
- PMID: 19934406
- PMCID: PMC2838521
- DOI: 10.1152/ajpendo.00477.2009
Protein kinase C: poised to signal
Abstract
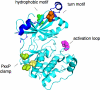
Nestled at the tip of a branch of the kinome, protein kinase C (PKC) family members are poised to transduce signals emanating from the cell surface. Cell membranes provide the platform for PKC function, supporting the maturation of PKC through phosphorylation, its allosteric activation by binding specific lipids, and, ultimately, promoting the downregulation of the enzyme. These regulatory mechanisms precisely control the level of signaling-competent PKC in the cell. Disruption of this regulation results in pathophysiological states, most notably cancer, where PKC levels are often grossly altered. This review introduces the PKC family and then focuses on recent advances in understanding the cellular regulation of its diacylglycerol-regulated members.
Figures


Similar articles
-
The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):69-81. doi: 10.11124/jbisrir-2015-2335. JBI Database System Rev Implement Rep. 2015. PMID: 26571284
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7. PMID: 38260445 Free PMC article. Updated. Preprint.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits.Biochemistry. 2008 Jul 22;47(29):7598-609. doi: 10.1021/bi8006803. Biochemistry. 2008. PMID: 18627125 Free PMC article. Review.
Cited by
-
The human biliverdin reductase-based peptide fragments and biliverdin regulate protein kinase Cδ activity: the peptides are inhibitors or substrate for the protein kinase C.J Biol Chem. 2012 Jul 13;287(29):24698-712. doi: 10.1074/jbc.M111.326504. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584576 Free PMC article.
-
Protein kinase Cɛ activity regulates mGluR5 surface expression in the rat nucleus accumbens.J Neurosci Res. 2017 Apr;95(4):1079-1090. doi: 10.1002/jnr.23868. Epub 2016 Aug 21. J Neurosci Res. 2017. PMID: 27546836 Free PMC article.
-
Molecular characterization of host-parasite cell signalling in Schistosoma mansoni during early development.Sci Rep. 2016 Oct 20;6:35614. doi: 10.1038/srep35614. Sci Rep. 2016. PMID: 27762399 Free PMC article.
-
Toward a biorelevant structure of protein kinase C bound modulators: design, synthesis, and evaluation of labeled bryostatin analogues for analysis with rotational echo double resonance NMR spectroscopy.J Am Chem Soc. 2015 Mar 18;137(10):3678-85. doi: 10.1021/jacs.5b00886. Epub 2015 Mar 4. J Am Chem Soc. 2015. PMID: 25710634 Free PMC article.
-
Biliverdin reductase: a target for cancer therapy?Front Pharmacol. 2015 Jun 3;6:119. doi: 10.3389/fphar.2015.00119. eCollection 2015. Front Pharmacol. 2015. PMID: 26089799 Free PMC article. Review.
References
-
- Akamine P, Madhusudan Wu J, Xuong NH, Ten Eyck LF, Taylor SS. Dynamic features of cAMP-dependent protein kinase revealed by apoenzyme crystal structure. J Mol Biol 327: 159–171, 2003 - PubMed
-
- Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res 67: 8828–8838, 2007 - PubMed
-
- Balendran A, Hare GR, Kieloch A, Williams MR, Alessi DR. Further evidence that 3-phosphoinositide-dependent protein kinase-1 (PDK1) is required for the stability and phosphorylation of protein kinase C (PKC) isoforms. FEBS Lett 484: 217–223, 2000 - PubMed
-
- Bartlett PJ, Young KW, Nahorski SR, Challiss RA. Single cell analysis and temporal profiling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors. J Biol Chem 280: 21837–21846, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

