SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro
- PMID: 19933795
- PMCID: PMC2812147
- DOI: 10.1128/AAC.00660-09
SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro
Abstract
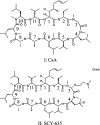
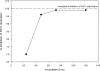
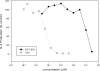
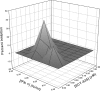
SCY-635 is a novel nonimmunosuppressive cyclosporine-based analog that exhibits potent suppression of hepatitis C virus (HCV) replication in vitro. SCY-635 inhibited the peptidyl prolyl isomerase activity of cyclophilin A at nanomolar concentrations but showed no detectable inhibition of calcineurin phosphatase activity at concentrations up to 2 microM. Metabolic studies indicated that SCY-635 did not induce the major cytochrome P450 enzymes 1A2, 2B6, and 3A4. SCY-635 was a weak inhibitor and a poor substrate for P-glycoprotein. Functional assays with stimulated Jurkat cells and stimulated human peripheral blood mononuclear cells indicated that SCY-635 is a weaker inhibitor of interleukin-2 secretion than cyclosporine. A series of two-drug combination studies was performed in vitro. SCY-635 exhibited synergistic antiviral activity with alpha interferon 2b and additive antiviral activity with ribavirin. SCY-635 was shown to be orally bioavailable in multiple animal species and produced blood and liver concentrations of parent drug that exceeded the 50% effective dose determined in the bicistronic con1b-derived replicon assay. These results suggest that SCY-635 warrants further investigation as a novel therapeutic agent for the treatment of individuals who are chronically infected with HCV.
Figures


Similar articles
-
The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes.Antimicrob Agents Chemother. 2012 Jul;56(7):3888-97. doi: 10.1128/AAC.00693-12. Epub 2012 May 14. Antimicrob Agents Chemother. 2012. PMID: 22585215 Free PMC article.
-
The cyclophilin inhibitor SCY-635 suppresses viral replication and induces endogenous interferons in patients with chronic HCV genotype 1 infection.J Hepatol. 2012 Jul;57(1):47-54. doi: 10.1016/j.jhep.2012.02.024. Epub 2012 Mar 13. J Hepatol. 2012. PMID: 22425702 Clinical Trial.
-
Potent nonimmunosuppressive cyclophilin inhibitors with improved pharmaceutical properties and decreased transporter inhibition.J Med Chem. 2014 Oct 23;57(20):8503-16. doi: 10.1021/jm500862r. Epub 2014 Oct 13. J Med Chem. 2014. PMID: 25310383
-
Cyclophilin inhibitors.Clin Liver Dis. 2009 Aug;13(3):403-17. doi: 10.1016/j.cld.2009.05.002. Clin Liver Dis. 2009. PMID: 19628157 Review.
-
Cyclophilin inhibitors for the treatment of HCV infection.Curr Opin Investig Drugs. 2010 Aug;11(8):911-8. Curr Opin Investig Drugs. 2010. PMID: 20721833 Review.
Cited by
-
Peptidyl-Proline Isomerases (PPIases): Targets for Natural Products and Natural Product-Inspired Compounds.J Med Chem. 2016 Nov 10;59(21):9622-9644. doi: 10.1021/acs.jmedchem.6b00411. Epub 2016 Jul 25. J Med Chem. 2016. PMID: 27409354 Free PMC article. Review.
-
Cyclophilins and cyclophilin inhibitors in nidovirus replication.Virology. 2018 Sep;522:46-55. doi: 10.1016/j.virol.2018.06.011. Epub 2018 Jul 12. Virology. 2018. PMID: 30014857 Free PMC article. Review.
-
Proline Isomerization: From the Chemistry and Biology to Therapeutic Opportunities.Biology (Basel). 2023 Jul 14;12(7):1008. doi: 10.3390/biology12071008. Biology (Basel). 2023. PMID: 37508437 Free PMC article. Review.
-
Domain 3 of NS5A protein from the hepatitis C virus has intrinsic alpha-helical propensity and is a substrate of cyclophilin A.J Biol Chem. 2011 Jun 10;286(23):20441-54. doi: 10.1074/jbc.M110.182436. Epub 2011 Apr 13. J Biol Chem. 2011. PMID: 21489988 Free PMC article.
-
Current update on theranostic roles of cyclophilin A in kidney diseases.Theranostics. 2022 May 13;12(9):4067-4080. doi: 10.7150/thno.72948. eCollection 2022. Theranostics. 2022. PMID: 35673572 Free PMC article. Review.
References
-
- Alpini, G., J. O. Phillips, B. Vroman, and N. F. Larusso. 1994. Recent advances in the isolation of liver cells. Hepatology 20:494-514. - PubMed
-
- Baumgrass, R., Y. Zhang, F. Erdmann, A. Thiel, M. Weiwad, A. Radbruch, and G. Fischer. 2004. Substitution in position 3 of cyclosporin A abolishes the cyclophilin-mediated gain-of-function mechanism but not immunosuppression. J. Biol. Chem. 279:2470-2479. - PubMed
-
- Billich, A., F. Hammerschmid, P. Peichl, R. Wenger, G. Zenke, V. Quesniaux, and B. Rosenwirth. 1995. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J. Virol. 69:2451-2461. - PMC - PubMed
-
- Edlich, F., and G. Fischer. 2006. Pharmacological targeting of catalyzed protein folding: the example of peptide bond cis/trans isomerases, p. 359-404. In M. Gaestel (ed.), Handbook of experimental pharmacology, vol. 172. Springer-Verlag, Berlin, Germany. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

