A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease
- PMID: 19923550
- PMCID: PMC2790221
- DOI: 10.1212/WNL.0b013e3181c67808
A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease
Abstract
Background: Bapineuzumab, a humanized anti-amyloid-beta (Abeta) monoclonal antibody for the potential treatment of Alzheimer disease (AD), was evaluated in a multiple ascending dose, safety, and efficacy study in mild to moderate AD.
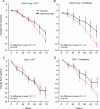
Methods: The study enrolled 234 patients, randomly assigned to IV bapineuzumab or placebo in 4 dose cohorts (0.15, 0.5, 1.0, or 2.0 mg/kg). Patients received 6 infusions, 13 weeks apart, with final assessments at week 78. The prespecified primary efficacy analysis in the modified intent-to-treat population assumed linear decline and compared treatment differences within dose cohorts on the Alzheimer's Disease Assessment Scale-Cognitive and Disability Assessment for Dementia. Exploratory analyses combined dose cohorts and did not assume a specific pattern of decline.
Results: No significant differences were found in the primary efficacy analysis. Exploratory analyses showed potential treatment differences (p < 0.05, unadjusted for multiple comparisons) on cognitive and functional endpoints in study "completers" and APOE epsilon4 noncarriers. Reversible vasogenic edema, detected on brain MRI in 12/124 (9.7%) bapineuzumab-treated patients, was more frequent in higher dose groups and APOE epsilon4 carriers. Six vasogenic edema patients were asymptomatic; 6 experienced transient symptoms.
Conclusions: Primary efficacy outcomes in this phase 2 trial were not significant. Potential treatment differences in the exploratory analyses support further investigation of bapineuzumab in phase 3 with special attention to APOE epsilon4 carrier status.
Classification of evidence: Due to varying doses and a lack of statistical precision, this Class II ascending dose trial provides insufficient evidence to support or refute a benefit of bapineuzumab.
Figures
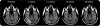
Comment in
-
APOE {epsilon}4 and bapineuzumab: Infusing pharmacogenomics into Alzheimer disease therapeutics.Neurology. 2009 Dec 15;73(24):2052-3. doi: 10.1212/WNL.0b013e3181c6784a. Epub 2009 Nov 18. Neurology. 2009. PMID: 19923549 No abstract available.
-
A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease.Neurology. 2010 Jun 15;74(24):2026; author reply 2026-7. doi: 10.1212/WNL.0b013e3181e03844. Neurology. 2010. PMID: 20548049 No abstract available.
Similar articles
-
Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease.N Engl J Med. 2014 Jan 23;370(4):322-33. doi: 10.1056/NEJMoa1304839. N Engl J Med. 2014. PMID: 24450891 Free PMC article. Clinical Trial.
-
Bapineuzumab for mild to moderate Alzheimer's disease in two global, randomized, phase 3 trials.Alzheimers Res Ther. 2016 May 12;8(1):18. doi: 10.1186/s13195-016-0189-7. Alzheimers Res Ther. 2016. PMID: 27176461 Free PMC article. Clinical Trial.
-
11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study.Lancet Neurol. 2010 Apr;9(4):363-72. doi: 10.1016/S1474-4422(10)70043-0. Epub 2010 Feb 26. Lancet Neurol. 2010. PMID: 20189881 Clinical Trial.
-
Bapineuzumab.Expert Opin Biol Ther. 2010 Jul;10(7):1121-30. doi: 10.1517/14712598.2010.493872. Expert Opin Biol Ther. 2010. PMID: 20497044 Free PMC article. Review.
-
Bapineuzumab: anti-β-amyloid monoclonal antibodies for the treatment of Alzheimer's disease.Immunotherapy. 2010 Nov;2(6):767-82. doi: 10.2217/imt.10.80. Immunotherapy. 2010. PMID: 21091109 Review.
Cited by
-
Vascular Considerations for Amyloid Immunotherapy.Curr Neurol Neurosci Rep. 2022 Nov;22(11):709-719. doi: 10.1007/s11910-022-01235-1. Epub 2022 Oct 21. Curr Neurol Neurosci Rep. 2022. PMID: 36269539 Free PMC article. Review.
-
Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele.PLoS One. 2012;7(7):e41636. doi: 10.1371/journal.pone.0041636. Epub 2012 Jul 25. PLoS One. 2012. PMID: 22848551 Free PMC article.
-
An MRI rating scale for amyloid-related imaging abnormalities with edema or effusion.AJNR Am J Neuroradiol. 2013 Aug;34(8):1550-5. doi: 10.3174/ajnr.A3475. Epub 2013 Feb 22. AJNR Am J Neuroradiol. 2013. PMID: 23436056 Free PMC article. Clinical Trial.
-
Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials.PLoS One. 2013;8(3):e58816. doi: 10.1371/journal.pone.0058816. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554933 Free PMC article.
-
The ADAS-Cog revisited: novel composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials.Alzheimers Dement. 2013 Feb;9(1 Suppl):S21-31. doi: 10.1016/j.jalz.2012.05.2187. Epub 2012 Nov 2. Alzheimers Dement. 2013. PMID: 23127469 Free PMC article.
References
-
- Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 2003;43:545–584. - PubMed
-
- Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000;6:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG005134/AG/NIA NIH HHS/United States
- P01 NS15655/NS/NINDS NIH HHS/United States
- 1U01AG032438-01/AG/NIA NIH HHS/United States
- P50 AG08671/AG/NIA NIH HHS/United States
- 1 R01 MH58620-01A2/MH/NIMH NIH HHS/United States
- P50AG08702/AG/NIA NIH HHS/United States
- R01AG017761/AG/NIA NIH HHS/United States
- R43AG033474/AG/NIA NIH HHS/United States
- R01AG14763/AG/NIA NIH HHS/United States
- 1-UO1-AG024904/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01AG026114/AG/NIA NIH HHS/United States
- G0801306/MRC_/Medical Research Council/United Kingdom
- AG08671/AG/NIA NIH HHS/United States
- 1RL1AA017536-01/AA/NIAAA NIH HHS/United States
- P01AG07232/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- AG06036-13/AG/NIA NIH HHS/United States
- U54RR024350-01/RR/NCRR NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U01-AG16976/AG/NIA NIH HHS/United States
- R01AG027435/AG/NIA NIH HHS/United States
- R01NS042859/NS/NINDS NIH HHS/United States
- 1 R03 AG023916-01A1/AG/NIA NIH HHS/United States
- UO1AGO24904/PHS HHS/United States
- R01HL091099/HL/NHLBI NIH HHS/United States
- P50MH068789/MH/NIMH NIH HHS/United States
- 1-R01-AG030457-01A1/AG/NIA NIH HHS/United States
- 5-U01-AG10483/AG/NIA NIH HHS/United States
- T32 NS007224-19/NS/NINDS NIH HHS/United States
- R01AG007370/AG/NIA NIH HHS/United States
- P20 RR020626/RR/NCRR NIH HHS/United States
- IR21DK062098/DK/NIDDK NIH HHS/United States
- P01 AG030004-01/AG/NIA NIH HHS/United States
- AF10483/AF/ACF HHS/United States
- P30 P3-AG 019610/AG/NIA NIH HHS/United States
- G0601846/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
