Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor
- PMID: 19922973
- PMCID: PMC3102050
- DOI: 10.1016/j.virol.2009.10.036
Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor
Abstract
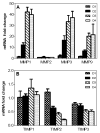
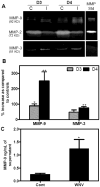
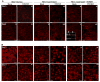
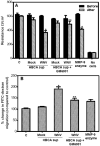
Though compromised blood-brain barrier (BBB) is a pathological hallmark of WNV-associated neurological sequelae, underlying mechanisms are unclear. We characterized the expression of matrix metalloproteinases (MMP) in WNV-infected human brain microvascular endothelial cells (HBMVE) and human brain cortical astrocytes (HBCA), components of BBB and their role in BBB disruption. Expression of multiple MMPs was significantly induced in WNV-infected HBCA cells. Naïve HBMVE cells incubated with the supernatant from WNV-infected HBCA cells demonstrated loss of tight junction proteins, which were rescued in the presence of MMP inhibitor, GM6001. Further, supernatant from WNV-infected HBCA cells compromised the in vitro BBB model integrity. Our data suggest astrocytes as one of the sources of MMP in the brain, which mediates BBB disruption allowing unrestricted entry of immune cells into the brain, thereby contributing to WNV neuropathogenesis. Because of the unavailability of WNV antivirals and vaccines, use of MMP inhibitors as an adjunct therapy to ameliorate WNV disease progression is warranted.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier.Virology. 2009 Mar 15;385(2):425-33. doi: 10.1016/j.virol.2008.11.047. Epub 2009 Jan 10. Virology. 2009. PMID: 19135695 Free PMC article.
-
West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases.J Gen Virol. 2012 Jun;93(Pt 6):1193-1203. doi: 10.1099/vir.0.040899-0. Epub 2012 Mar 7. J Gen Virol. 2012. PMID: 22398316 Free PMC article.
-
Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat.J Cereb Blood Flow Metab. 2007 Apr;27(4):697-709. doi: 10.1038/sj.jcbfm.9600375. Epub 2006 Jul 19. J Cereb Blood Flow Metab. 2007. PMID: 16850029
-
Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia.Neurosurg Focus. 2007 May 15;22(5):E4. doi: 10.3171/foc.2007.22.5.5. Neurosurg Focus. 2007. PMID: 17613235 Review.
-
Blood-brain barrier abnormalities caused by HIV-1 gp120: mechanistic and therapeutic implications.ScientificWorldJournal. 2012;2012:482575. doi: 10.1100/2012/482575. Epub 2012 Feb 1. ScientificWorldJournal. 2012. PMID: 22448134 Free PMC article. Review.
Cited by
-
In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium.PLoS One. 2016 Jun 23;11(6):e0157786. doi: 10.1371/journal.pone.0157786. eCollection 2016. PLoS One. 2016. PMID: 27336851 Free PMC article.
-
Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis.Neurotherapeutics. 2016 Jul;13(3):514-34. doi: 10.1007/s13311-016-0443-5. Neurotherapeutics. 2016. PMID: 27220616 Free PMC article. Review.
-
Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses.Viruses. 2023 Jan 17;15(2):261. doi: 10.3390/v15020261. Viruses. 2023. PMID: 36851477 Free PMC article. Review.
-
West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins.PLoS One. 2012;7(5):e37886. doi: 10.1371/journal.pone.0037886. Epub 2012 May 24. PLoS One. 2012. PMID: 22655077 Free PMC article.
-
A Journey to the Central Nervous System: Routes of Flaviviral Neuroinvasion in Human Disease.Viruses. 2022 Sep 21;14(10):2096. doi: 10.3390/v14102096. Viruses. 2022. PMID: 36298652 Free PMC article. Review.
References
-
- Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol. 2007;179(4):2576–83. - PubMed
-
- Amantea D, Russo R, Gliozzi M, Fratto V, Berliocchi L, Bagetta G, Bernardi G, Corasaniti MT. Early upregulation of matrix metalloproteinases following reperfusion triggers neuroinflammatory mediators in brain ischemia in rat. Int Rev Neurobiol. 2007;82:149–69. - PubMed
-
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23(5):406–15. - PubMed
-
- Arjona A, Ledizet M, Anthony K, Bonafe N, Modis Y, Town T, Fikrig E. West Nile Virus Envelope Protein Inhibits dsRNA-Induced Innate Immune Responses. J Immunol. 2007b;179(12):8403–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

